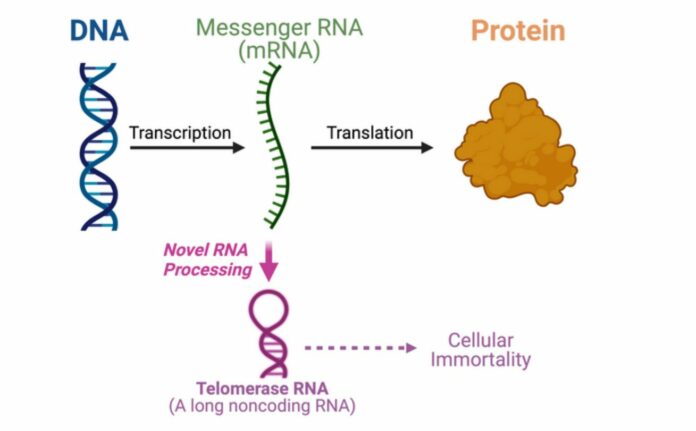
The Center for the Mechanism of Evolution at the Biodesign Institute and the School of Molecular Sciences at Arizona State University, under the direction of Julian Chen, have for the first time found an entirely unexpected route that converts a messenger RNA (mRNA) that codes for protein into telomerase RNA.
The core dogma of molecular biology lays out the steps that must be taken from DNA to produce a protein. Messenger RNA molecules transport genetic data from the DNA in the cell’s nucleus to the cytoplasm, which is where proteins are produced. Messenger RNA is the way that proteins are made.
“Actually, there are many RNAs (ribonucleic acids) that are not used to make proteins,” says Chen. “About 70 percent of the human genome is used to make noncoding RNAs that don’t code for protein sequences but have other uses.”
One of the non-coding RNAs that comes together with the telomerase proteins to generate the enzyme telomerase is telomerase RNA. For cancer and stem cells to be immortal, telomerase is essential. In this study, Chen’s team demonstrates that a fungal telomerase RNA is processed from a protein-encoding mRNA rather than being produced independently.
“Our finding from this paper is paradigm-shifting. Most RNA molecules are synthesized independently and here we uncovered a dual function mRNA that can be used to produce a protein or to make a noncoding telomerase RNA, which is really unique,” Chen adds. “We will need to do a lot more research to understand the underlying mechanism of such an unusual RNA biogenesis pathway.”
Basic research on how mRNA is used and how it is controlled has led to important medical uses. Several COVID-19 vaccines, for instance, employ messenger RNA to create viral spike proteins. In these vaccines, the mRNA molecules break down over time, and then our bodies take them in.
Compared to DNA vaccinations, which carry the risk of becoming permanently and negatively incorporated into human DNA, this new technique has advantages. This study’s finding of dual-function mRNA biogenesis may lead to novel approaches for developing mRNA vaccines in the future.
In this study, Chen’s team was surprised to find telomerase RNA made from mRNA in the model fungus Ustilago maydis, also known as corn smut. Eating corn smut, also known as Mexican truffle, gives many foods, such as tamales and tacos, a delightful umami flavor.
Finding new pathways for mRNA metabolism and telomerase biogenesis may be possible by studying the biology of RNA and telomeres in corn smut.
Why should telomerase RNA be studied?
The Nobel Prize in Physiology or Medicine was awarded in 2009 “for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase.” Telomerase was first isolated from a unicellular organism living in pond scum. As it later turned out, telomerase exists in almost all eukaryotic organisms, including humans, and plays a crucial role in aging and cancer. Scientists have been scrambling to discover ways to utilize telomerase to make human cells immortal.
Typical human cells are mortal and cannot forever renew themselves. As demonstrated by Leonard Hayflick a half-century ago, human cells have a limited replicative life span, with older cells reaching this limit sooner than younger cells. This “Hayflick limit” of cellular life span is directly related to the number of unique DNA repeats found at the ends of the genetic material-bearing chromosomes. These DNA repeats are part of the protective capping structures, termed “telomeres,” which safeguard the ends of chromosomes from unwanted and unwarranted DNA rearrangements that destabilize the genome.
Each time the cell divides, the telomeric DNA shrinks and will eventually fail to secure the chromosome ends. This continuous reduction of telomere length functions as a “molecular clock” that counts down to the end of cell growth.
The diminished ability for cells to grow is strongly associated with the aging process, with the reduced cell population directly contributing to weakness, illness and organ failure.
Counteracting the telomere shrinking process is telomerase, the enzyme that uniquely holds the key to delaying or even reversing the cellular aging process. Telomerase offsets cellular aging by lengthening the telomeres, adding back lost DNA repeats to add time onto the molecular clock countdown, effectively extending the life span of the cell.
Telomerase lengthens telomeres by repeatedly synthesizing very short DNA repeats of six nucleotides — the building blocks of DNA — with the sequence “GGTTAG” onto the chromosome ends from a template located within the RNA component of the enzyme itself.
The gradual shrinking of telomeres negatively affects the replicative capacity of human stem cells, the cells that restore damaged tissues and/or replenish aging organs in our bodies. The activity of telomerase in adult stem cells merely slows down the countdown of the molecular clock and does not completely immortalize these cells. Therefore, adult stem cells become exhausted in aged individuals due to telomere length shortening which results in increased healing times and organ tissue degradation from inadequate cell populations.
Tapping the full potential of telomerase
Understanding the regulation and limitation of the telomerase enzyme holds the promise of reversing telomere shortening and cellular aging with the potential to extend human life span and improve wellness of elderly individuals.
Human diseases that include dyskeratosis congenita, aplastic anemia and idiopathic pulmonary fibrosis have been genetically linked to mutations that negatively affect telomerase activity and/or accelerate the loss of telomere length. This accelerated telomere shortening closely resembles premature aging with increased organ deterioration and a shortened patient life span caused by critically insufficient stem cell populations. Increasing telomerase activity is seemingly the most promising means of treating these genetic diseases.
While increased telomerase activity could bring youth to aging cells and cure premature aging-like diseases, too much of a good thing can be damaging for the individual. Just as youthful stem cells use telomerase to offset telomere length loss, cancer cells employ telomerase to maintain their aberrant and destructive growth. Augmenting and regulating telomerase function will have to be performed with precision, walking a narrow line between cell rejuvenation and a heightened risk for cancer development.
Distinct from human stem cells, somatic cells constitute the vast majority of the cells in the human body and lack telomerase activity. The telomerase deficiency of human somatic cells reduces the risk of cancer development, as telomerase fuels uncontrolled cancer cell growth. Therefore, drugs that increase telomerase activity indiscriminately in all cell types are not desired. Small molecule drugs can be screened or designed to increase telomerase activity exclusively within stem cells for disease treatment as well as antiaging therapies without increasing the risk of cancer.
The study of telomerase RNA biogenesis in corn smut may unveil new mechanisms for telomerase regulation and offer new directions on how to modulate or engineer human telomerase for innovations in developing antiaging and anticancer therapeutics.
Image Credit: Julian Chen
You were reading: “A Really Unique” Dual-function Messenger RNA Discovered