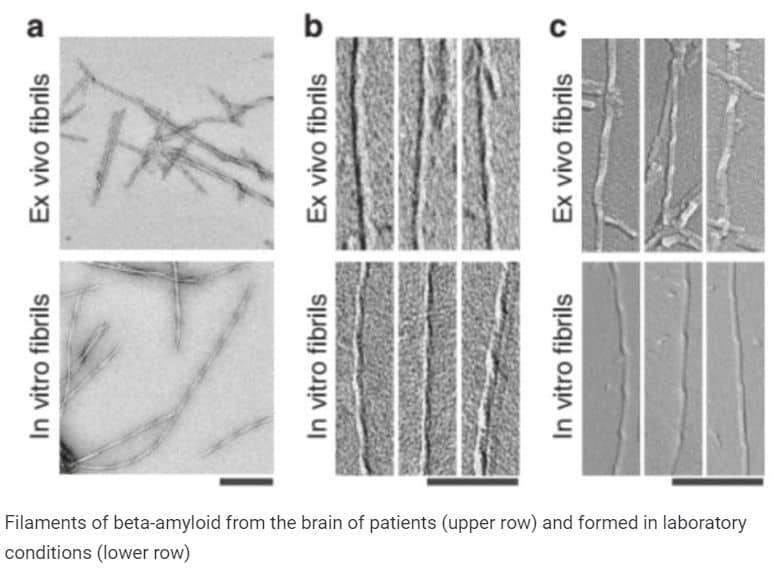
The strings of amyloid-beta from the brain of patients with Alzheimer’s disease were unlike the strings that it forms in the laboratory. This was announced today by a group of scientists who examined protein aggregates in an electron microscope. Unlike the laboratory counterpart, the amyloid from the patient’s brain is not twisted to the left, but to the right, and takes not so many different forms. This means that the models with which they were still looking for a cure for the disease may not be entirely true.
At the molecular level, Alzheimer’s disease is caused by the aggregation of two proteins: amyloid-beta and tau protein. But if tau aggregates are also found in other diseases (for example, in the brains of patients with Parkinson’s disease), accumulations of amyloid-beta are characteristic mainly of Alzheimer’s disease. It is believed that it is these proteins that lead to the death of nerve cells, but most often their aggregation is studied in vitro.
Marius Kollmer and his colleagues from Australia, Germany and the United States decided to characterize amyloid-beta aggregates in real brain tissue. To do this, they took tissue samples of the soft membranes of the brain of three patients who died from Alzheimer’s disease.
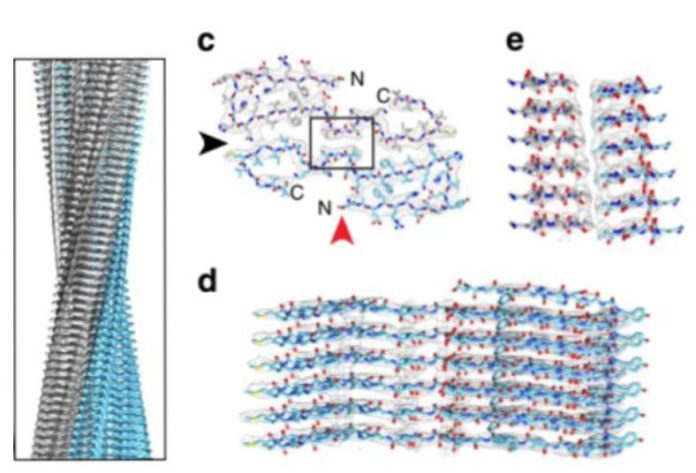
Having examined the amyloid filaments in a transmission electron microscope, the researchers noticed that they have a spiral structure that resembles in shape aggregates formed in vitro, but not twisted to the left, but to the right. In addition, the “real” strands were more stable than their laboratory counterparts and did not decompose under the action of protease enzymes.
Kollmer et al. Nature Communications (2019)
Scientists also found using cryoelectronic microscopy that the vast majority of the strands that they isolated from the patient’s brain belong to three types of structure, depending on the thickness of the strand and the frequency of twisting of the spiral. Three types were equally found in all three samples, so they cannot be called patient-specific. At the same time, in previous in vitro studies, amyloid-beta took many different forms. This probably means that in real tissue the spectrum of possible amyloid structures is very limited or depends on the specific subtype of the disease.
So far, among the scientists who deal with Alzheimer’s disease, the “amyloid hypothesis” has prevailed, according to which the cause of the disease is the accumulation of amyloid-beta, and the cure for it should destroy protein aggregates or interfere with their assembly. However, not a single drug that the researchers created for this has shown efficacy in clinical trials.
The authors of the new work cautiously note that their data do not allow us to say unequivocally that the amyloid forms completely different structures in vivo and in vitro, but indicate that laboratory models do not always accurately reflect reality. In fact, this means that, while developing tools to combat amyloid beta aggregates, scientists still did not very well imagine what their target looks like.
Previously, scientists have already suggested that Alzheimer’s disease be considered not a single disease, but a group of diseases, based on the structure of amyloid-beta. It has also been suggested that its aggregation serves to protect the brain from infections.