Parkinson’s Disease Drug Ropinirole Trial for Amyotrophic Lateral Sclerosis (ALS)
New Hope For People With Amyotrophic Lateral Sclerosis (ALS) As Parkinson’s Disease Drug Ropinirole Found To Improve Daily Activity And Muscle Strength and Delay Disease Progression By 27.9 Weeks On Average
Amyotrophic lateral sclerosis (ALS), also referred to as Lou Gehrig’s disease, is a progressive neurodegenerative disorder that leads to the gradual loss of muscle control. It is a fatal condition with no known cure, and the current approach to treatment focuses on alleviating symptoms and providing supportive care.
In a new study published today in the journal Cell Stem Cell, Japanese researchers have demonstrated the safety of using the Parkinson’s disease drug ropinirole in ALS patients through an early clinical trial. Notably, the trial showed that ropinirole extended the average duration of disease progression by approximately 27.9 weeks.
Interestingly, the response to ropinirole treatment varied among patients, with certain individuals exhibiting greater responsiveness. Additionally, the researchers were able to predict the clinical effectiveness of ropinirole in vitro by utilizing motor neurons derived from stem cells obtained from ALS patients.
“ALS is totally incurable, and it’s a very difficult disease to treat,” remarks senior author and physiologist Hideyuki Okano.
“We previously identified ropinirole as a potential anti-ALS drug in vitro by iPSC drug discovery, and with this trial, we have shown that it is safe to use in ALS patients and that it potentially has some therapeutic effect, but to confirm its effectiveness we need more studies, and we are now planning a phase 3 trial for the near future.”
In order to assess the safety and efficacy of ropinirole in individuals with sporadic ALS (non-familial ALS), a group of researchers enlisted the participation of 20 patients receiving treatment at Keio University Hospital in Japan. None of these patients carried genetic predispositions to the disease, and, on average, they had been living with ALS for a duration of 20 months.
During the trial, the first 24 weeks were conducted as a double-blind study, meaning that both the patients and doctors were unaware of whether they were receiving ropinirole or a placebo. Subsequently, for the subsequent 24 weeks, all patients who chose to continue with the study were knowingly administered ropinirole. The trial encountered several dropouts along the way, with the COVID-19 pandemic being a contributing factor. As a result, only 7 out of 13 patients who received ropinirole treatment and 1 out of 7 patients who initially received a placebo followed by ropinirole treatment were monitored for the entire year. Importantly, none of the patients dropped out due to safety concerns.
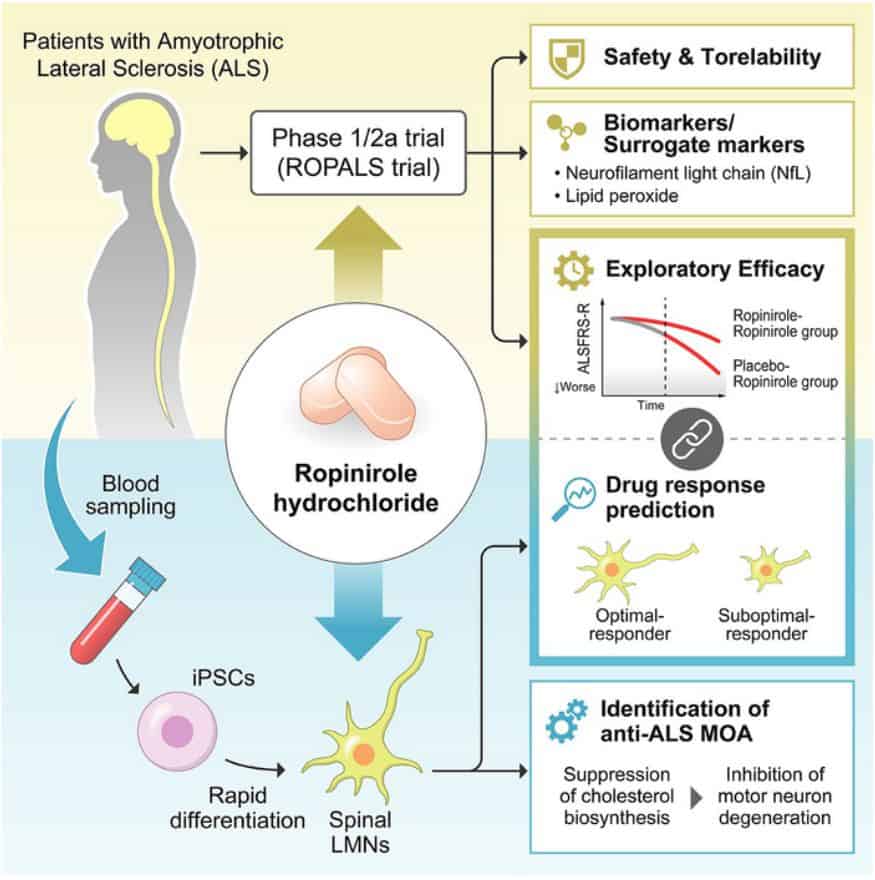
To evaluate the drug’s effectiveness in slowing down the progression of ALS, the research team conducted regular assessments throughout the trial and for an additional 4 weeks after the treatment was concluded. These assessments encompassed various measures, such as self-reported changes in physical activity and the ability to independently eat and drink, data collected from wearable devices regarding activity levels, as well as evaluations conducted by physicians to measure alterations in mobility, muscle strength, and lung function.
“We found that ropinirole is safe and tolerable for ALS patients,” adds first author Satoru Morimoto, “and shows therapeutic promise at helping them sustain daily activity and muscle strength.”
Patients who received ropinirole throughout both phases of the trial exhibited higher levels of physical activity compared to those in the placebo group. Additionally, they demonstrated a slower decline in mobility, muscle strength, and lung function, and they had a higher likelihood of survival.
The advantages of ropinirole over the placebo became more evident as the trial progressed. However, patients in the placebo group who started taking ropinirole halfway through the trial did not experience the same improvements. This suggests that ropinirole treatment may be more effective when initiated earlier and administered over a more extended period.
Subsequently, the researchers delved into the mechanisms underlying the effects of ropinirole and sought molecular markers of the disease. To accomplish this, they generated induced pluripotent stem cells from the patients’ blood and cultivated these cells into motor neurons in the laboratory. In comparison to healthy motor neurons, the motor neurons derived from ALS patients exhibited distinct differences in structure, gene expression, and metabolite concentrations. However, treatment with ropinirole reduced these disparities.
Specifically, motor neurons developed from ALS patients displayed shorter neurites than their healthy counterparts, but when these cells were treated with ropinirole, the axons grew to a more normal length. The research team also identified 29 genes associated with cholesterol synthesis that tended to be upregulated in motor neurons derived from ALS patients. However, ropinirole treatment gradually suppressed the expression of these genes over time. Furthermore, they discovered that lipid peroxide served as a reliable surrogate marker for estimating the impact of ropinirole both in vitro and clinically.
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” adds Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression despite taking ropinirole.”
According to the researchers, this finding indicates that the approach of cultivating and evaluating motor neurons derived from induced pluripotent stem cells obtained from patients could have clinical applications in predicting the effectiveness of the drug for individual patients. While the reasons behind varying responsiveness to ropinirole among patients remain unclear, the researchers hypothesize that genetic differences may play a significant role. They intend to investigate and identify these genetic factors in future studies.
Image Credit: Getty