Neurological disorders ranging from anxiety to schizophrenia and Parkinson’s disease to autistic spectrum disorder may have new solutions according to USC Dornsife researchers employing cryogenic electron microscopy as a diagnostic tool.
For the first time, scientists have deciphered the atomic structure of a protein that transports one of the body’s most vital neurotransmitters into neurons.
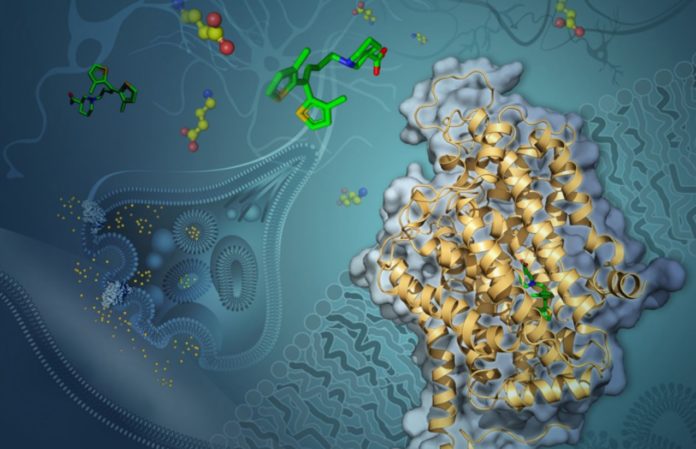
By determining the structure of this transporter protein — one of the smallest proteins ever resolved — researchers have opened up new avenues for improving treatments for a variety of debilitating conditions, including epilepsy, bipolar disorder, schizophrenia, Parkinson’s and Huntington’s diseases, anxiety, and autism spectrum disorders.
The research was published in the journal Nature today.
Neurons communicate with one another by sending neurotransmitters via synapses, which are gaps between them. The neurotransmitter GABA (short for gamma-amino butyric acid) is one of the most common in the brain.
When a neuron releases GABA and sends it across a synapse to a neighbouring neuron, GABA inhibits the receiving cell’s activity.
However, there are situations when not enough GABA reaches the receiving neuron, causing it to become overactive and send too many electrical impulses. This can have a variety of negative consequences, including seizures.
Fortunately, medications that block a protein called GAT-1 can help (short for GABA transporter 1). GAT-1 is in charge of reintroducing released GABA into the emitting neuron.
Tiagabine (brand name Gabitril), a GAT-1 inhibitor, inhibits GABA recycling, leaving more GABA in the synapse to diminish receiving neuron activity.
Although tiagabine is effective, the exact mechanism by which it inhibits GABA recycling by interacting with GAT-1 is unknown. Knowing how they interact could help researchers develop more effective medications in the future.
To unravel the enigma and determine how tiagabine binds to GAT-1, researchers from the USC Dornsife College of Letters, Arts, and Sciences using cryo-EM, a highly advanced form of cryogenic electron microscopy. The method entails freezing molecules at extremely low temperatures, close to the point at which atoms and molecules cease to move, and then photographing them with an electron microscope.
USC recently showcased its new cryo-EM laboratory within the USC Michelson Center for Convergent Bioscience’s Core Center of Excellence in Nano Imaging.
Cornelius Gati, the study’s lead author, and a group of USC Dornsife researchers observed GAT-1 in complex with tiagabine and used cryo-EM to show how the two interact.
“Previous understanding of the inhibitor (tiagabine) was purely based on biochemical studies, which do not provide any details at the atomic scale,” Gati, an assistant professor of biological sciences and chemistry at USC Dornsife, explains. “We can now pinpoint specific parts of the drug that interact with the protein,” says the researcher.
This new revelation, according to Gati, points to a previously undiscovered mechanism of GAT-1 inhibition involving a change in the protein’s overall structure when tiagabine binds to it.
The study’s highly detailed information could aid researchers in improving medications or developing novel remedies for disorders involving GABA-controlled neurons.
“These findings have direct implications on pharmacology as a whole, not only with respect to treating epilepsy, but many other diseases,” Gati said, adding that the findings hint to new research directions.
“We have a feeling that this newly revealed mechanism is much more common than is currently thought, so we will investigate this first by using structurally similar inhibitors, and then expand our search to other molecules.”
Gati also mentioned that utilizing cryo-EM to resolve the structure of the interacting molecules was incredibly difficult – GAT-1 is one of the tiniest proteins resolved using the technique, making it tough to visualize even with such advanced technology.
Their breakthrough, he added, will inspire scientists at USC and other institutions to figure out the structures of additional difficult membrane proteins, resulting in a better knowledge of drug-protein interactions and more effective therapies.
Image Credit: USC Dornsife/Yekaterina Kadyshevskaya
You were reading: For the First Time, A Small Protein Structure Tied To All Brain Disorders – New Research