Alveoli are microscopic air sacs in the lungs that play a crucial role in the transfer of gases from the air we breathe into the bloodstream. To facilitate this process, the epithelial cells of the alveoli create a material known as “surfactant” that forms a film over the alveoli.
This complex is mostly made up of phospholipids and proteins, and its job is to lower the surface tension of the alveoli. Additionally, it serves as a filter, effectively capturing germs and viruses that are inhaled into the lungs.
Since alveolar macrophages (AMs), which are scavenger cells on the alveoli, constantly degrade and take away the chemical used as surfactant, the body is constantly secreting new surfactant to replace it.
By doing so, the body is able to keep its homeostasis, or stable state, between surfactant synthesis and disposal. Professor Alexander Mildner, a former Heisenberg fellow at the Max Delbrück Center and currently a group leader at the University of Turku, explains that if anything goes wrong, more and more of the secretion accumulates in the lungs, which affects breathing and raises the risk of lung infections.
The study’s final author, Mildner, has been studying macrophages for 20 years.
“We wanted to know what prevents these pulmonary phagocytes from functioning properly,” he adds.
Pulmonary alveolar proteinosis (PAP) is caused by an excessive buildup of surfactant and is currently incurable; in severe cases, patients’ lungs must be flushed on a regular basis to prevent further damage.
The critical function of C/EBPb
The study was started when it was found that alveolar macrophages can’t grow normally if they don’t have C/EBPb. For many years, Professor Achim Leutz has been studying how this transcription factor works.
At the MDC, he is in charge of the lab where Mildner conducted his own research—the Cell Differentiation and Tumorigenesis Lab. In addition to Dr. Uta Höpken and Dr. Daro Jess Lupiáez Garca, other MDC researchers contributed to the study.
Animal trials and molecular biological analyses helped scientists determine what part C/EBPb plays. Their findings were just published in the journal Science Immunology.
Dr. Dorothea Dörr, the study’s principal author, adds, “We isolated alveolar macrophages from healthy mice and from those lacking the gene for C/EBPb and conducted in vitro tests on these immune cells.”
Additionally, they analyzed the genome and transcriptome of freshly isolated cells. The study focused on the biological and molecular characteristics of AMs, namely their capacity for lipid absorption and metabolism.
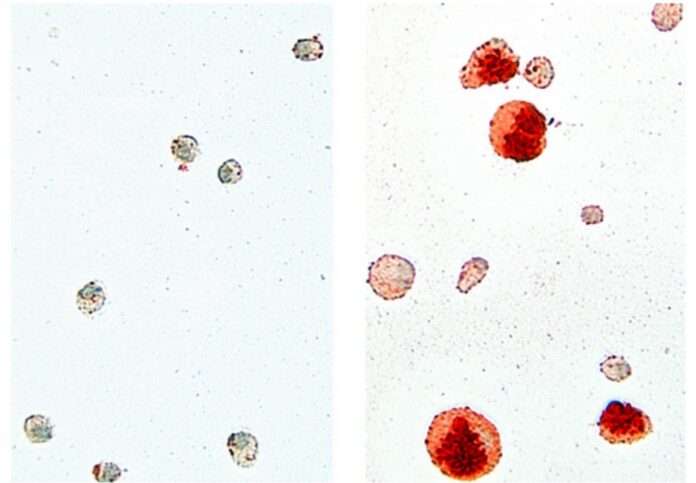
Unlike the healthy mice’s macrophages, which carried out their tasks as intended, the genetically altered mice’s macrophages absorbed and retained a lot of the lipids but were unable to break down them.
Instead, they grew into so-called “foam cells” and quickly died, redepositing the lipids they had previously consumed. Doctors treating PAP lung disease have noted the same phenomenon. Also, the macrophages with problems were hardly able to multiply.
An essential component of the puzzle
Molecular investigations revealed that another essential gene, PPARg, which is also a transcription factor, is downregulated in mice lacking the C/EBPb gene. When active, this promotes the body’s fatty acid absorption as well as the differentiation of fat cells and macrophages.
The lung condition PAP is typically caused by issues with the cytokine GM-signaling CSF’s pathway, which stands for granulocyte-macrophage colony-stimulating factor.
“We already knew that certain essential functions of alveolar macrophages are controlled via the GM-CSF signaling pathway,” adds Mildner. “Now we have found that macrophages deficient in C/EBPb show severe malfunctions in the proliferation of these cells and the degradation of surfactant, causing a PAP-like pathology in mice.”
Therefore, it appears that the missing regulatory link between the GM-CSF and PPARg signaling pathways is C/EBPb.
Leutz says, “It’s like a jigsaw puzzle.” If you insert a specific piece, finding other missing pieces becomes much simpler.
Unlocking other diseases’ mysteries
Although macrophages are known as the immune system’s scavenger cells, they perform a variety of other functions as well. Each organ has its own unique type of macrophage.
For instance, they are tasked with destroying neurons and synapses that are no longer required during the remodeling of the brain. They risk developing disorders of the central nervous system if they don’t complete this task properly.
In addition to being the primary factor in PAP, abnormal lipid metabolism is also the cause of the deadly vascular disease atherosclerosis.
During this disease, fat builds up on the walls of the arteries, where white blood cells called macrophages trap it. These macrophages consume the lipids, but because they are unable to properly break them down, they enlarge and create plaques.
If the plaques ever rupture, the fat that is inside may escape and form artery-blocking clots, which might result in a heart attack or stroke.
According to Mildner, “We think that the signaling pathway we have shed light on could be important in many lipid-related diseases.
According to Mildner, “We think that the signaling pathway we have shed light on could be important in many lipid-related diseases. “So the question now is whether what we’ve learned from alveolar macrophages might also help us better understand atherosclerosis and morbid obesity (adiposity).”
“So the question now is whether what we’ve learned from alveolar macrophages might also help us better understand atherosclerosis and morbid obesity (adiposity).”
PAP may soon have a new treatment. It is well known that several therapeutic drugs have the ability to alter PPARg. It might be able to restart the lipid metabolism of dysregulated alveolar macrophages when combined with a C/EBPb-activating medication.
Source: 10.1126/sciimmunol.abj0140
Image Credit: AG Leutz, MDC
You were reading: New Findings Reveal What Happens When Macrophage Digestion Goes Wrong