Researchers at the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University have discovered how a tiny cellular machine called TRiC controls the folding of tubulin, a human protein that is the key component of microtubules, which serve as the cell’s scaffolding and transport system.
Researchers previously believed that TRiC as well as other elements known as chaperonins passively create an environment that promotes folding without actively taking part in it.
The researchers calculated that up to 10% of the proteins in human cells, as well as those of plants and animals, get assistance from these tiny chambers in folding into their final, active forms.
Many of the proteins that fold with TRiC’s help are associated to human illnesses like as cancer and neurological disorders such as Parkinson’s, Huntington’s, and Alzheimer’s, according to Stanford Professor Judith Frydman, one of the study’s primary authors.
She said that many anti-cancer drugs are made to mess up tubulin and the microtubules it makes, which are very important for cell division. Therefore, focusing on the TRiC-assisted tubulin folding process may provide a promising anti-cancer approach.
Their decade-long investigation was published in Cell today.
“This is the most exciting protein structure I have worked on in my 40-year career,” says SLAC/Stanford Professor Wah Chiu.
“When I met Judith 20 years ago,” he adds, “we talked about whether we could see proteins folding. That’s something people have been trying to do for years, and now we have done it.”
With cryo-EM, the researchers were able to see four distinct steps in the TRiC-directed folding process, and biochemical and biophysical tests confirmed what they saw.
The finding, according to Frydman, “really is a game changer in finally bringing a new way to understand how proteins fold in the human cell,” since it resolves the long-standing mystery of why tubulin can’t fold without TRiC’s help.
Flower-folding spaghetti
Proteins are important for almost everything a cell does, and figuring out how they fold into their final 3D shapes is one of the most important goals in chemistry and biology.
According to Chiu, a protein first appears as a spaghetti-like strand of amino acids but cannot perform its intended function until it has been folded into the precise shape of a flower.
Since the middle of the 1950s, tests conducted on tiny proteins by National Institutes of Health researcher Christian Anfinsen have changed our understanding of how proteins fold. He found that if he unfolded a small protein, it would spring back into the same shape on its own. He concluded that the protein’s amino acid sequence held the instructions for how to do this. For this discovery, Anfinsen received a part of the 1972 Nobel Prize in Chemistry.
Thirty years later, scientists learned that certain cellular machinery aids in the folding of proteins. However, the prevailing theory was that their role was confined to preventing proteins from being caught or clumping together when they spontaneously folded.
A chaperonin is a type of helper machine that has a chamber that looks like a barrel and holds proteins while they fold. This is where TRiC fits in.
The TRiC chamber is one of a kind since it is made up of eight distinct components that stack on top of each other to create two rings. A jellyfish-shaped aid molecule transports a long, slender strand of tubulin protein into the chamber’s entrance. Then folding starts when the chamber’s lid shuts. The lid opens to release the folded tubulin.
Since tubulin can’t fold without TRiC, it seemed like TRiC did more than just help tubulin fold on its own. But how does it really operate? This new research provides a response to that query and shows that the “spontaneous folding” idea does not hold true, at least not for proteins like tubulin. Instead, TRiC directly directs the protein folding process that results in the desired protein structure.
Frydman noted that although AI has advanced to the point where it can accurately predict the folded structure of most proteins, this still doesn’t reveal the steps involved in folding. This insight is crucial for managing cellular folding and creating treatments for folding-related illnesses. To do this, scientists must understand the precise steps taken by the folding process as it takes place within the cell.
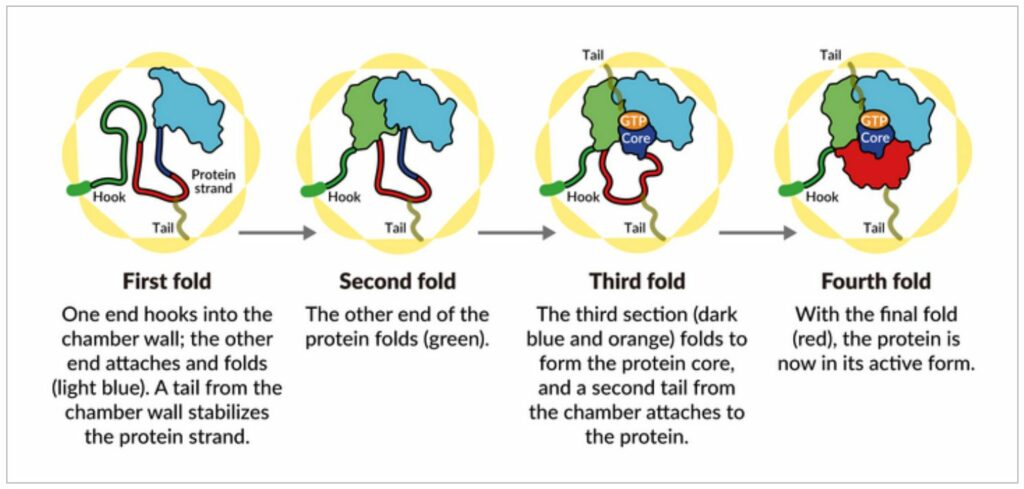
A cell chamber runs things
Frydman, Chiu, and the members of their respective research teams made the decision to look more closely at what occurs within the TRIC chamber ten years ago.
“Compared to the simpler folding chambers of chaperonins in bacteria, the TRiC in human cells is a very interesting and complicated machine,” Frydman adds. “Each of its eight subunits has different properties and presents a distinct surface inside the chamber, and this turns out to be really important.”
Scientists found that this unique chamber’s interior can direct the folding process in two different ways.
Areas of electrostatic charge emerge on the inside walls of the chamber when the lid shuts over a protein. They pull together parts of the tubulin protein strand that have opposite charges and “tack” them to the wall. This gives the protein the right shape and arrangement for the next step of folding. The tubulin protein is anchored and stabilized by TRiC component “tails” that hang from the chamber wall at certain times and locations.
The tubulin strand starts by hooking onto a wall recess at one end. As a result, the other end folds in and connects at a new location. The wall-hooked end folds close to the initial folded section.
In step three, a part of the middle section folds to make the protein’s center and pockets for GTP, which stores and releases energy to power the cell’s work.
Finally, the last piece of protein folds. The molecule of tubulin is now ready to do its job.
“These structural snapshots of intermediate stages in the folding sequence have never been seen before by cryo-electron microscopy,” remarks Frydman.
A powerful mix of ways to do things
Her team confirmed the folding sequence by putting it through a difficult set of biochemical and biophysical tests that took years to complete.
By analyzing those findings, the researchers were able to create a model that matched the pictures produced by cryo-EM and depicted how the tubulin changes form as it folds within the TRiC chamber.
“It’s very powerful to be able to go back and forth between these techniques, because then you can really know that what you see reflects what’s going on in the cell,” Frydman adds.
“Science has surprised us with a really interesting solution that I would not have predicted.”
The study also gives hints about how this folding system evolved in eukaryotic cells, which are found in plants, animals, and humans, but not in simpler cells like those of bacteria and archaea. The researchers hypothesize that as proteins became more sophisticated to meet the demands of eukaryotic cells, they eventually lost their ability to fold into the forms required to perform increasingly difficult tasks on their own. The last common ancestor of all eukaryotic species, which lived around 2.7 billion years ago, may have served as the beginning point for the evolution of eukaryotic proteins and associated chaperonin chamber.
Source: 10.1016/j.cell.2022.11.014