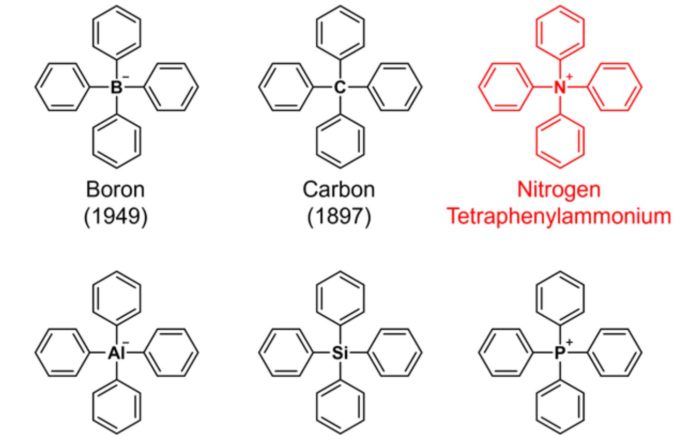
Tetraphenylammonium, which has all four ammonium (NH4+) hydrogens substituted with benzene rings, has yet to be detected in nature or chemically synthesized, raising doubts about its existence.
For the first time, scientists were able to synthesize tetraphenylammonium, confirming its stable existence.
The radical coupling synthetic method adopted in this study may be useful for the synthesis of numerous related ammoniums with great structural originality.
A structure consisting just of a common element and the benzene ring is regarded as one of the most fundamental chemical skeletons, as the benzene ring is a typical component of organic molecules.
The chemical synthesis of such compounds has been investigated from the beginning of organic chemistry because of their importance.
The structure in which four benzene rings are connected to a representative element (boron, carbon, aluminum, silicon, or phosphorus) of groups 13 to 15 in the periodic table (Fig. 1), for example, was synthesized more than 70 years ago, with the oldest synthetic report dating back 137 years.
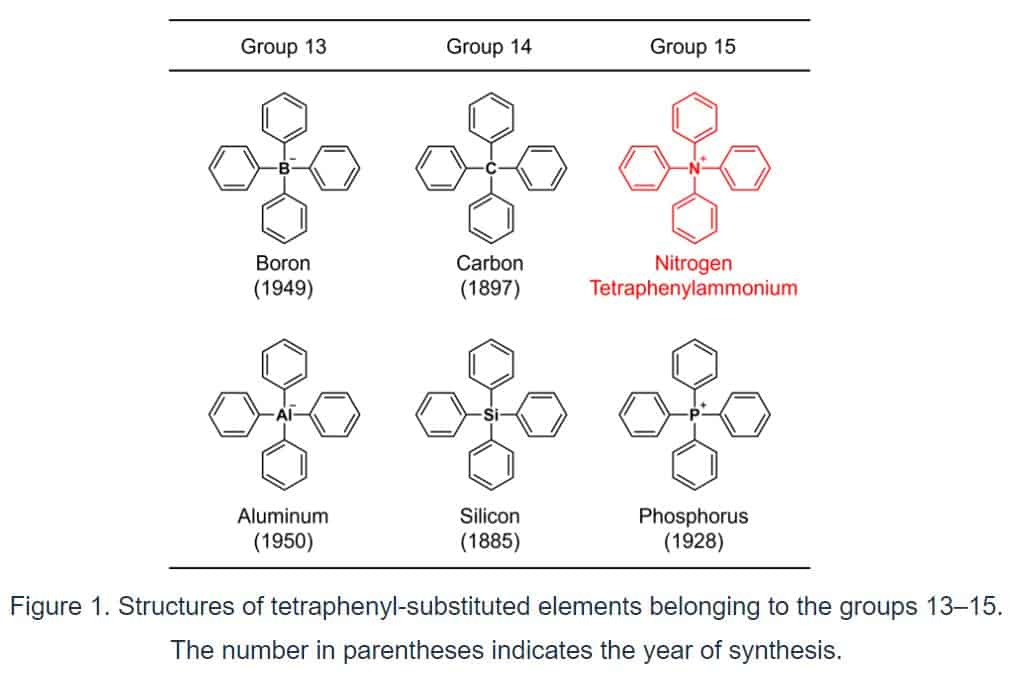
By adding “tetra” meaning “4” to “phenyl”*1), these skeletons are generally referred to as “tetraphenyl” structures, indicating that they contain four benzene rings. When the core element is nitrogen, the parent ion is ammonium (NH4+).
Tetraphenylammonium is an example of such a chemical.
This compound, which is actually an ion, has a relatively straightforward chemical structure that even a novice in organic chemistry may comprehend. Nonetheless, creating this structure artificially has proven to be extremely challenging, and no synthetic reports with clear structural identification have been published.
Furthermore, because tetraphenylammonium has never been observed in nature, it has been unclear whether it can exist at all. There have been publications that assume its existence and just describe its application without explaining its synthesis or acquisition method. Only the chemical structure is stored in compound databases.
As a result, this ion is occasionally referred to as if it were already well-known. Tetraphenylammonium, on the other hand, has never been seen, making it a “phantom ion.”
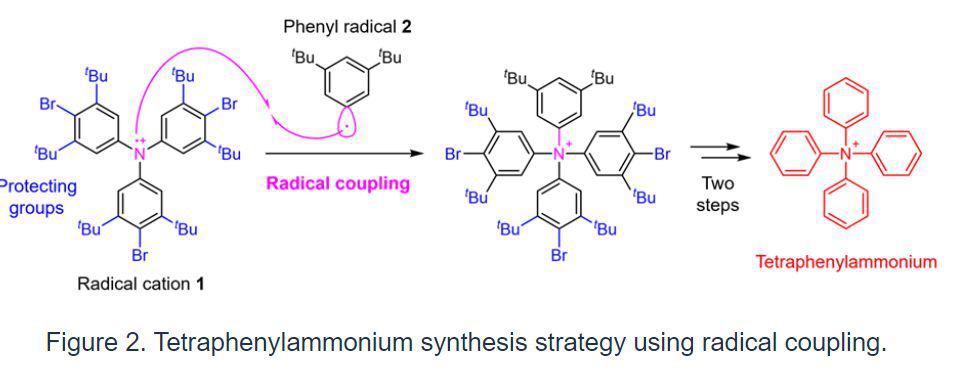
A research team from Kanazawa University’s Faculty of Pharmaceutical Sciences established a novel synthetic approach to enable the synthesis of tetraphenylammonium in this work. The attachment of the fourth phenyl group to the nitrogen atom, which already has three phenyl groups attached, is the crucial step in the synthesis of tetraphenylammonium.
This synthesis was supposed to be difficult to obtain using traditional methods. As a result, the researchers adopted a technique known as radical coupling2) and a strategy of reacting the radical cation 1 generated from a triphenylamine derivative with the phenyl radical 2 in the current work (Fig. 2).
As a result, the study team was able to complete the chemical conversion even if the yield was as low as 0.1 percent.
In such radical couplings, highly reactive radicals make bonds with one other, facilitating bond formation that could not be achieved by other means. On the other hand, because the reactivity is excessively high, it is difficult to manage selectivity, resulting in a variety of side reactions. As a result, the research team created the introduction of protective groups3) that produce steric hindrance in this synthesis in order to suppress as much as possible the side reaction of bond formation on the carbon of radical cation 1.
In the last step, a known triphenylamine derivative, which was the starting point for the synthesis, was changed chemically in five steps by adding protecting groups, radical coupling, and then removing the protecting groups, which led to tetraphenylammonium.
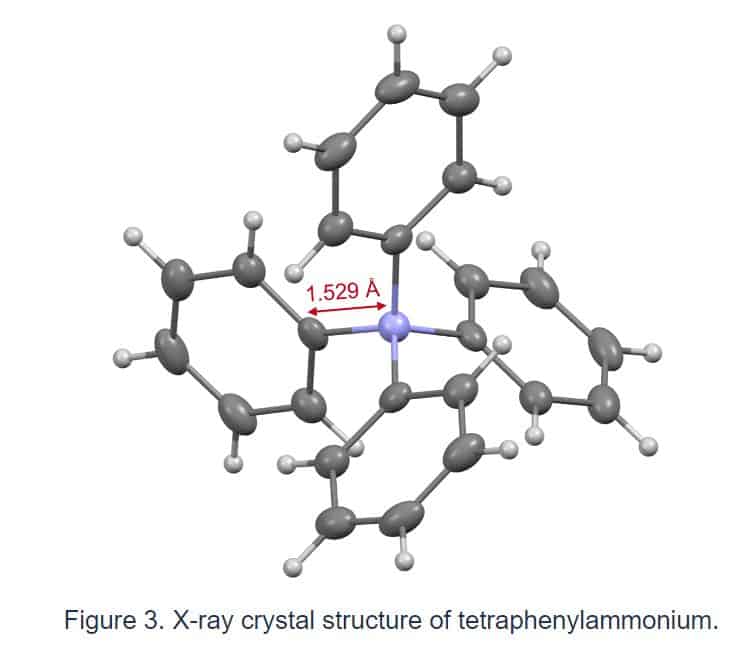
The structure of tetraphenylammonium was validated using data from multiple instrumental analyses. X-ray crystallography*4) showed that the length of the bond between the nitrogen atom and the carbon atom in the phenyl group of this ion is only 1.529. (Fig. 3).
Because this link is shorter than that of a tetraphenyl structure containing another element (boron, carbon, aluminum, silicon, or phosphorus), the nitrogen atom of tetraphenylammonium is clearly in a more spatially hindered environment than other elements. One of the elements that makes constructing this skeleton tough is this three-dimensional impediment. Furthermore, research findings demonstrated that tetraphenylammonium has a good stability in both acidic and basic environments.
The current research has shown that tetraphenylammonium exists and can be chemically produced. If large-scale production of this ion and its derivatives is achieved in the future, it could be used as an organic cation with great chemical stability in a variety of scientific domains. Furthermore, the radical coupling technique used in this study could be applied to synthesize other related ammoniums that had hitherto been unable to make.
Image Credit: Getty
You were reading: Scientists Confirm Existence Of Ghost Ion For The First Time