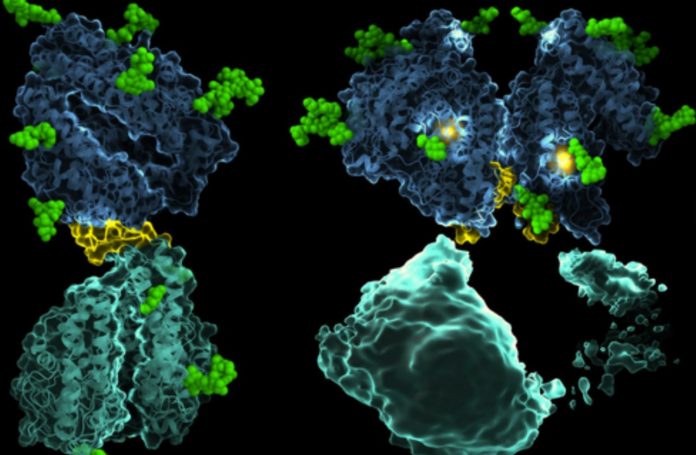
Using cryo-electron microscopy (cryo-EM), researchers from the University of Cape Town (UCT) have established the first detailed structures of the human angiotensin-converting enzyme (ACE). ACE is a protein that regulates blood pressure and is essential for heart health.
The cryo-EM structures of ACE in two alternative conformations, which will be published in The EMBO Journal today, have the potential to improve medicine design for cardiovascular disease, the leading cause of mortality worldwide.
The study was funded by a Global Challenges Research Fund grant from the UK Research and Innovation’s Science and Technology Facilities Council (STFC) as part of the START (Synchrotron Techniques for African Research and Technology) initiative.
The study was carried out by Dr. Lizelle Lubbe, Dr. Jeremy Woodward, Prof. Ed Sturrock, and Prof. Trevor Sewell. The Sturrock Laboratory at UCT synthesized the ACE protein, and the Electron Microscope Unit (EMU) at UCT processed it for high-resolution imaging.
It was moved to the UK’s national synchrotron, Diamond Light Source (Diamond), for high-resolution imaging at the electron Bio-Imaging Centre (eBIC). Both the EMU and the CSIR Centre for High Performance Computing (CHPC) in South Africa were used for image processing.
Since it produces the hormone Angiotensin II, which constricts blood vessels and increases blood pressure, ACE is an important target for the treatment of hypertension (high blood pressure) and heart disease.
Heart failure, heart attacks, kidney disease, strokes, and eyesight loss are all greatly increased by hypertension. It is frequently asymptomatic and is referred to as “the silent killer.”
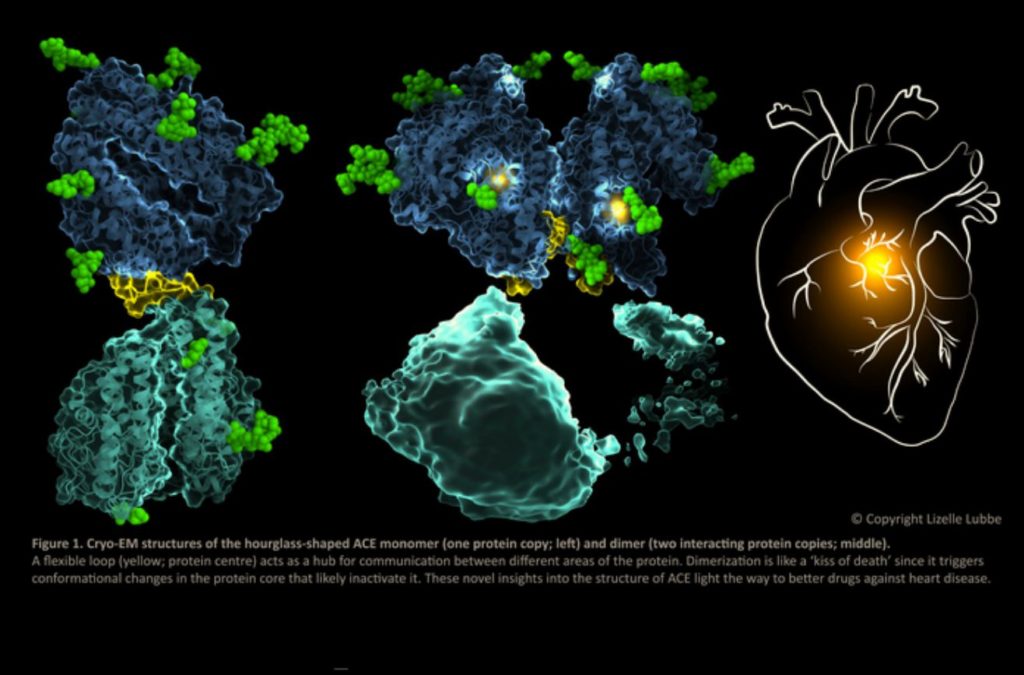
ACE’s monomeric form (one copy of the protein) is interesting because it is made up of two linked domains that have the same structure but different functions.
The study revealed that it also exists in a functionally important dimeric form (two interacting protein copies).
The function of ACE and its drug binding characteristics, which are essential for therapeutic drug design, are influenced by communication between the various sections of ACE.
According to Professor Sturrock, the study’s principal investigator, ACE inhibitors are clinically indicated as one of the first-line treatments for hypertension, but they non-selectively target both ACE domains and consequently cause side effects in some people.
Understanding the structure and dynamics of these newly observed variants of ACE is critical because it could help uncover novel areas for the construction of domain-selective inhibitors that avoid such adverse effects.
The results of the study provide a novel insight into the extremely dynamic nature of ACE and the mechanisms underlying dimerization and communication across its various domains.
As explained by Dr. Lubbe, study’s first author, by shifting from an active-site focused to a holistic view of this essential protein, they “obtained valuable new insights into how ACE works”.
Study co-author Professor Sewell adds, ACE’s dynamic nature hinders crystal formation, which meant that X-ray crystallography investigations over the past two decades could only solve sections of the structure.
“We discovered many clues using this method but were unable to solve the full puzzle until we had access to high-resolution cryo-EM.”
To extract the protein’s entire structure, it was rapidly cooled to -180 degrees Celsius, trapping the multiple conformations in a very thin, glass-like layer of water at the EMU.
After that, imaging was done at eBIC using a cutting-edge Titan Krios microscope. According to Dr. Woodward, a co-author of the paper, “even with high-resolution imaging, the unique shape, small size, and dynamic nature of ACE posed many challenges.”
“Recently developed cryo-EM image processing methods were crucial to solving the structures,” Dr. Lubbe adds. “We had to separate the images computationally through extensive classification, amounting to ‘digital purification’ because biochemical methods failed to separate the monomeric and dimeric forms of ACE. We could then solve both ACE structures by focusing the 3D refinement on different parts of the structure in turn.”
Image Credit: Lizelle Lubbe
You were reading: The First Full cryo-EM Structures of ACE Offer New Options For Killer Heart Diseases