Researchers have found that polystyrene, from which polystyrene is made, is completely or partially decomposed by sunlight. They conducted experiments and considered that completely polymer degrades in several centuries, and partially – in several decades.
Polystyrene began to be made in the 1960s and now tens of millions of tons of this polymer are produced in the world. It accounts for about six percent of global plastic production. Polystyrene is made of polystyrene, packaging for food products, children’s toys, used in construction, medicine and the military industry. It was first discovered in the ocean in the early 1970s and is regularly found now.
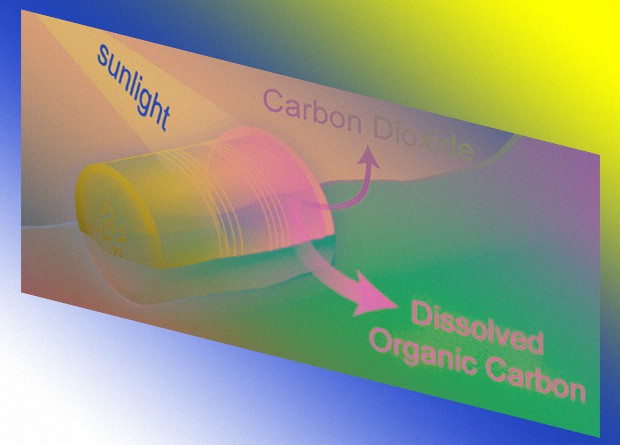
Scientists see the problem with polystyrene waste as it decomposes very slowly. This polymer is 75 percent aromatic compounds and has a high molecular weight, so microorganisms from the environment poorly break it. The researchers of one of the studies generally came to the conclusion that microbial communities do not act on it – they did not show signs of decomposition of polystyrene for several months. But, as it turned out, this polymer is partially oxidized by sunlight. However, the speed and completeness of this process are poorly understood. For example, it is not known whether polystyrene can completely decompose as a result of photochemical oxidation.
Therefore, Collin P. Ward of the Woods Hall Oceanographic Institute and his colleagues decided to see how this polymer behaves under the influence of sunlight. Scientists have taken five samples of polystyrene produced by American companies. They had different thicknesses, densities, and molecular weights, and during production different substances were added to them in order to achieve the desired physical or chemical properties. The polymers were placed in a sunlight simulator, the radiation intensity of which was 3-10 times higher than the intensity of natural sunlight at latitudes from zero to 50 degrees north latitude. The researchers chose these coordinates because they contain 10 large rivers that currently bring 90 percent of polystyrene waste into the ocean. Scientists considered the amount of oxygen that was absorbed during the reaction, and the amount of carbon dioxide and soluble carbon compounds that formed as a result of photochemical oxidation. They also looked at what wavelengths and what temperature the polymer degradation is faster.
All polystyrene samples completely or almost completely decomposed under the influence of sunlight (wavelength greater than 280 nanometers) at different time intervals. The completeness of polymer degradation and the wavelength at which it decomposed depended on the substances that were added to it during production. They absorbed different amounts of sunlight and, due to this, shortened or slowed down the oxidation process. The degradation rate of the polymer increased by about 25 percent (P = <0.05) with a temperature increase of ten degrees Celsius.
The authors calculated how long it would take for the two polystyrene samples on which they conducted the experiments to fully or partially decompose under natural conditions. According to them, the complete decomposition of samples should not take thousands of years, but hundreds of years, and partial tens. According to the authors, the half-life of samples with complete degradation is 100 and 300 years, and with partial degradation about 10 and 50 years.
“Now people who are developing recommendations for the use of plastic, suggest that polystyrene remains in the environment forever,” says Colleen Ward. “With this argument, they justify the creation of rules that completely prohibit it. Our goal was to understand whether polystyrene really remains in the environment forever. We are not saying that there is nothing wrong with plastic contamination, but the environmental polystyrene resistance may be less … than we previously thought. And the likelihood that the polymer will be harmful for decades is still there.”
A few years ago, scientists showed that expanded polystyrene is able to eat the larvae of the large flour hruschak. Bacteria in the intestines of the worms convert the polymer into carbon dioxide and biodegradable mass.
Source | Research Papers