A team of researchers led by Artem Oganov experimentally obtained a new high-temperature superconductor, thorium hydride ThH 10, and experimentally measured its properties. The resulting compound remains stable at a record low pressure of about 0.85 million atmospheres and retains superconducting properties at temperatures below 160 Kelvin and magnetic fields weaker than 45 Tesla. In addition, scientists measured the properties of several more thorium hydrides randomly synthesized with ThH 10.
For a long time, the title of the most “heat-resistant” superconductors was held by cuprates. First, these compounds were the first superconductors in history to retain their properties at temperatures above the boiling point of liquid nitrogen. Secondly, the record of the HgBa 2 Ca 2 Cu 3 O 8 x +, synthesized in the 1993 Year and rolling a superconducting state at a temperature of 164 Kelvin (-109 degrees Celsius), kept for more than twenty years.
Be that as it may, in 2015 this record broke a fundamentally new compound – ordinary hydrogen sulfide, compressed to the pressure of 1.5 million atmospheres. It turned out that in such extreme conditions, hydrogen sulfide passes into a superconducting state, which is stored when heated to 203 kelvins (-70 degrees Celsius). Moreover, shortly after the discovery of hydrogen sulfide superconductivity, theorists predicted a number of hydrides, which at comparable pressures turn into high-temperature Superconductors. To date, scientists have experimentally confirmed that hydrides of phosphorus, yttrium, cerium, uranium and lanthanum have similar properties, the latter of which is transformed into a superconductor at a temperature of about 260 kelvins (-13 degrees Celsius). Unfortunately, all these compounds remain stable only at extremely high pressures of about a million atmospheres. Therefore, despite the high critical temperature, close to the room, in practice, these superconductors can not be used.
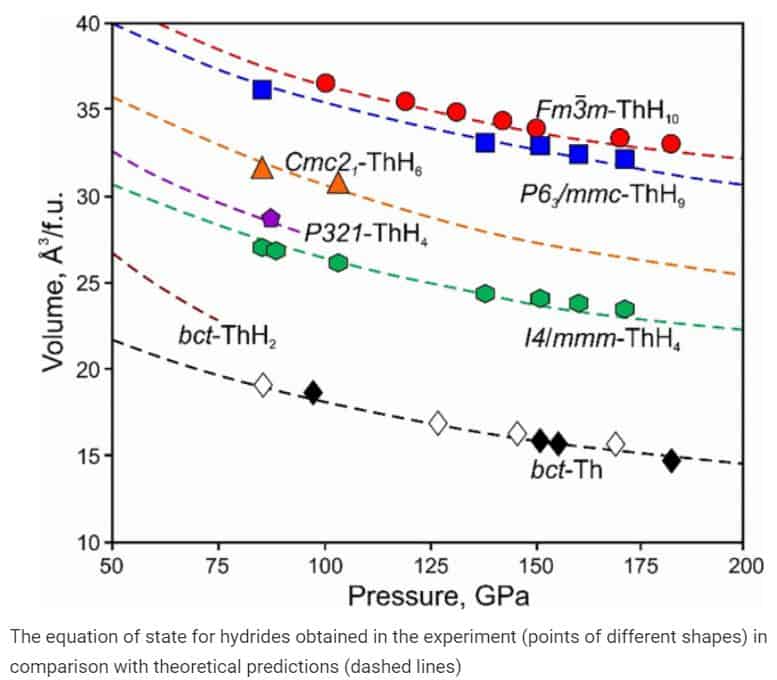
Last year, scientists have already researched this connection theoretically using the USPEX (Universal Structure Predictor: Evolutionary (X) Crystallography) algorithm. Then physicists found that the critical temperature of this superconductor at 20 degrees does not reach the record, but to create it you need the lowest pressure among all (ThH10 “falls apart” at a pressure below 0.8 million atmospheres). This made thorium hydride one of the most promising superconducting hydrides.
Now scientists have experimentally confirmed the predicted properties. To synthesize thorium hydride, physicists loaded a mixture of thorium and borazan into a diamond anvil cell with tungsten gasket. Using this anvil, the researchers compressed the sample to 1.7 million atmospheres and then warmed up to 1,800 kelvins using four laser pulses. The crystal structure of the resulting physics sample was determined by X-ray structural analysis. The scientists then slowly lowered the pressure in the cell and measured the sample temperature to restore its state equation. In general, the resulting crystal structure and state equation coincided with theoretical predictions. As expected, up to the pressures of about 0.85 million atmospheres, the connection remained stable.
To measure critical temperature and critical magnetic field, the scientists repeated the experiment in a slightly modified form. First, before compression, the researchers squeezed a sample between the golden-coated tantalum electrodes. Secondly, to isolate the sample from the external electric field, physicists inserted a layer of magnesium oxide into the gasket. Otherwise, the procedure of obtaining a hydride was not much different from the previous experience. After the sample was obtained, the scientists cooled it until the resistance fell to zero. The researchers repeated the same measurements for the non-zero external magnetic field. Unfortunately, this time the experiment quite diverged from the theory: at zero external magnetic field, the obtained critical temperature of the sample was 160 kelvin, that was one and a half times lower than the predicted value (240 kelvin). At the same time, the critical magnetic field (45 Tesla) generally coincided with the theory (38 Tesla).
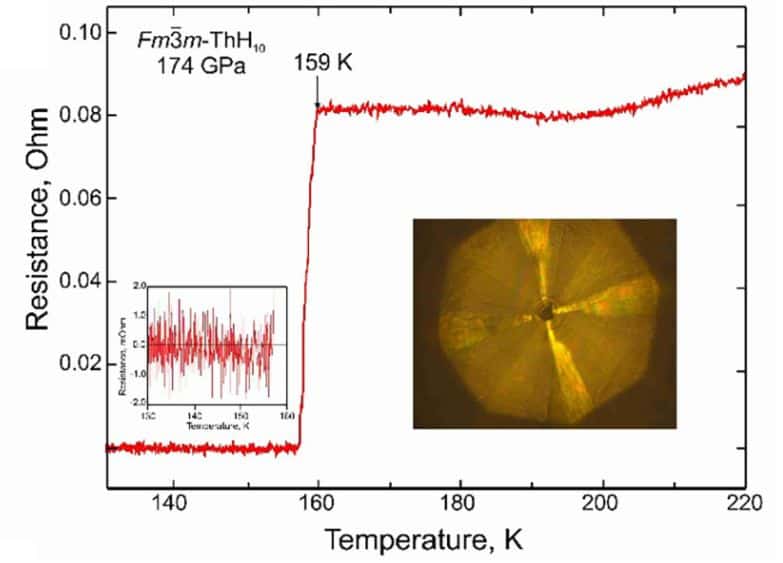
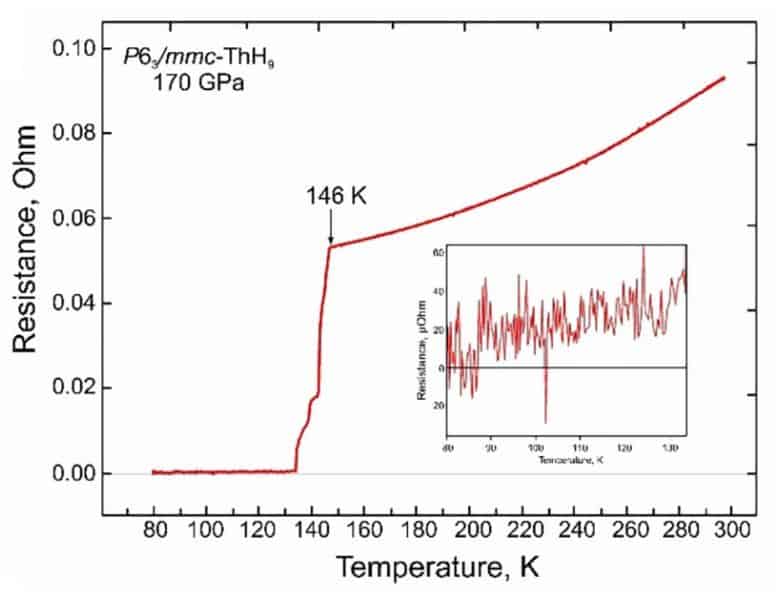
Image from Material Today
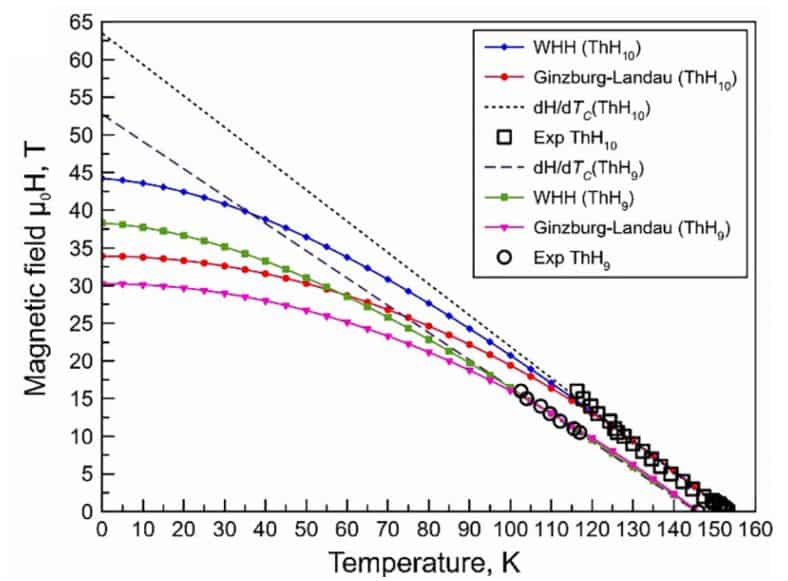
Image from Material Today
In addition, scientists repeated the same measurements for other thorium hydrides randomly synthesized during the experiment. One of these compounds, ThH 9 hydride, also turned out to be a superconductor, although less promising: under similar conditions, its critical temperature was 146 kelvin, and the critical magnetic field was 38 Tesla. In addition, it collapsed faster (ThH 9 “ fell apart” at a pressure of the order of a million atmospheres). For the two remaining compounds, the hydrides ThH 4 and ThH 6, the scientists measured the equation of state and determined the critical pressure below which the compounds begin to break down (0.86 and 1.04 million atmospheres, respectively). These compounds did not possess superconducting properties.
Oganov’s team has been working on the USPEX algorithm since 2004, during which time scientists have managed to predict many unusual substances produced at high pressures. In particular, with the help of this algorithm chemists have developed a new super-solid material, have shown that at high pressures nitric oxide acquires superconducting properties, and helium forms a stable compound with sodium, and is also found to be “impossible” in classical chemistry of forms of aluminium oxide, sodium chloride, magnesium, silicon and oxygen compounds. Most of the predicted connections have already been obtained in practice.