The entire world is closely following the development and testing of COVID-19 vaccines. The Russian Sputnik V, developed by the Gamaleya Center, and the British one from Oxford and AstraZeneca, are among the leaders. While the Russian vaccine is based on human adenoviruses, the British one is based on a chimpanzee adenovirus.
The Russian Direct Investment Fund (RFPI) compared vaccines based on human adenoviruses, those against chimpanzee adenoviruses and those based on mRNA in terms of the extent of their study.
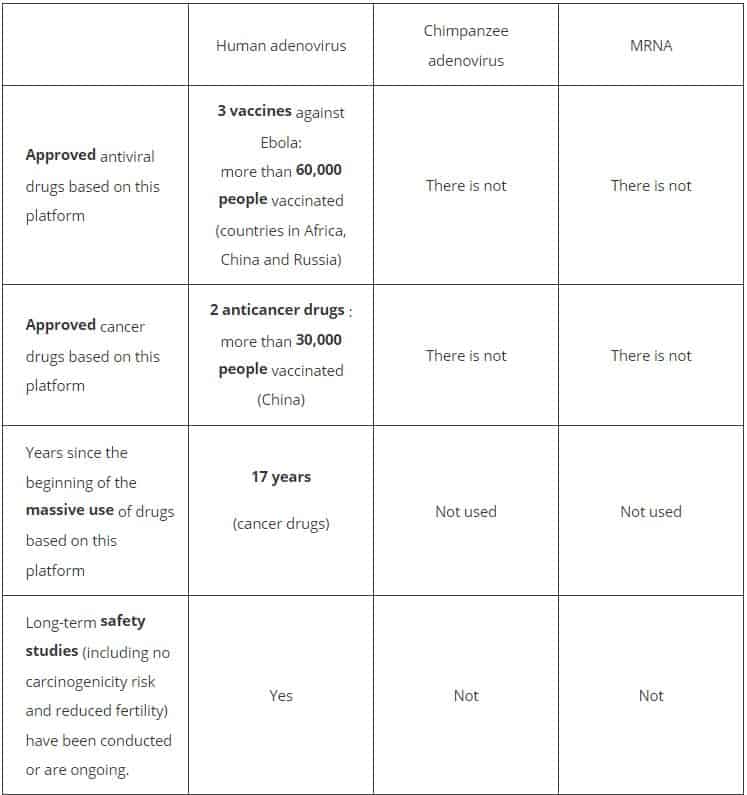
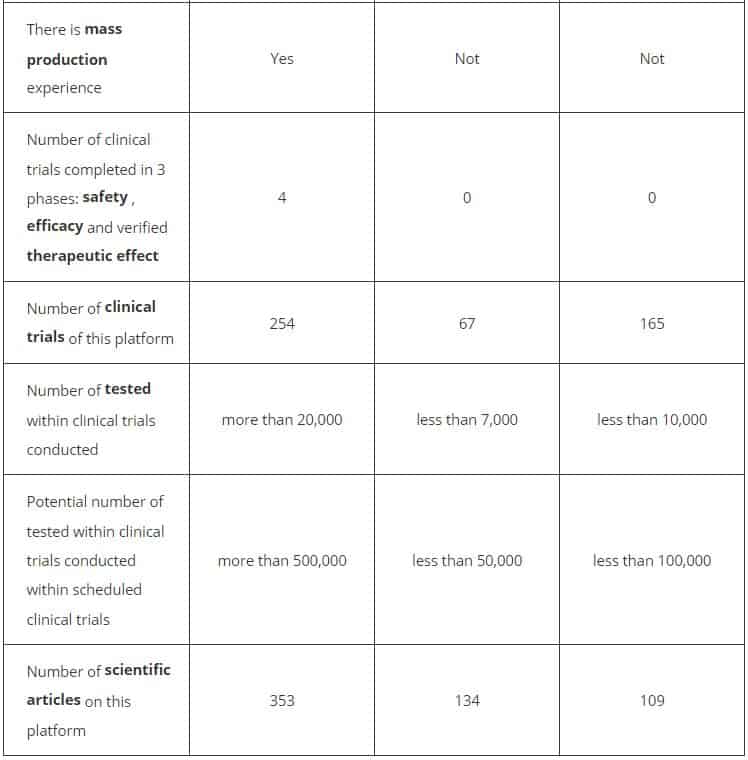
The RFPI also presented the key data on the study of human adenoviruses and the use of drugs developed on their basis:
- Research on human adenoviruses as a potential basis for vaccine production began in 1953.
- The vaccines do not contain live human adenoviruses, but their vectors. That is human viruses that cannot reproduce in the body and are completely safe for health.
- More than 20,000 people participated in clinical research on vaccines and preparations based on human adenoviruses or human adenovirus vectors.
- The most massive use of human adenovirus vaccines in the world is taking place in the US Armed Forces from 1971 to the present. The US Food and Drug Administration (FDA) approved human adenovirus vaccines in 2011. More than 10 million US soldiers received human adenovirus vaccines.
- A cancer drug based on human adenovirus vectors was used to treat more than 30,000 patients in China.
- Human adenovirus-based vaccines have been shown to pose no long-term health risks, carcinogenicity risks, or affect fertility. Health safety has been confirmed in more than 75 international publications and in more than 250 clinical trials.
According to the RFPI release, other means of delivering virus genetic material to stimulate the body’s immune response, such as chimp adenovirus or mRNA technology, have never before been used in approved vaccines. There have been no long-term studies on the possible effects of these technologies on the human body, including the possibility of cancer development and the effects on fertility.
Human adenovirus vectors are used in the development of coronavirus vaccines by the world’s leading pharmaceutical companies. However, their vaccines are single vector: CanSino uses the Ad5 vector, the use of which has already been approved by the Chinese Army; Johnson & Johnson, the vector Ad26. Johnson & Johnson received orders for more than 140 million doses of its drug from the United States and Europe.
According to the RFPI, the Gamaleya Research Institute of Epidemiology and Microbiology approach has a clear advantage because it uses two human adenovirus vectors of serotype 5 (Ad5) and 26 (Ad26).
Next week, Russia will launch a post-registration clinical trial of the Sputnik V vaccine in parallel with the vaccination of volunteers in risk groups. More than 40,000 people will participate in the study in more than 45 medical centers.
According to the director-general of RFPI, Kiril Dmitriev, the tests “will fully comply with international standards” and are “randomized, double-blind, placebo-controlled clinical trials.”
Sputnik V vaccine trials will begin in other countries in late August or early September.
