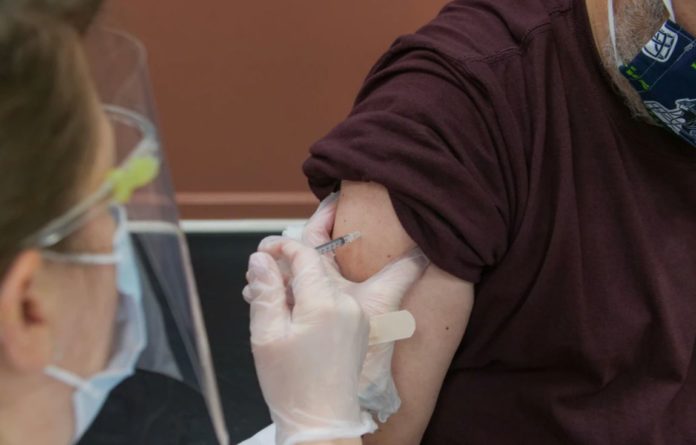
Ireland should temporarily suspend its Covid Vaccination Campaign with the Oxford/AstraZeneca jab after some reports found blood clotting in vaccinated people, health authorities said.
A statement issued by Ireland’s Deputy Chief Medical Officer, Dr. Ronan Glynn, stated: “Following new information received from the Norwegian Medicines Agency on Saturday evening 13th March and following discussions with the Health Products Regulatory Authority (HPRA), the National Immunisation Advisory Committee (NIAC) has recommended that the administration of COVID-19 Vaccine AstraZeneca be temporarily deferred from this morning, Sunday 14th March.
- Does This Mean We Stopped Being Animal and Started Being Human Due to ‘Copy Paste’ Errors?
- The One Lifestyle Choice That Could Reduce Your Heart Disease Risk By More Than 22%
- Aging: This Is What Happens Inside Your Body Right After Exercise
- Immune-Boosting Drink that Mimics Fasting to Reduce Fat – Scientists ‘Were Surprised’ By New Findings
- Gun Violence in America: What They Don’t Talk About at the Debate
“This recommendation has been made following a report from the Norwegian Medicines Agency of four new reports of serious blood clotting events in adults after vaccination with COVID-19 vaccine AstraZeneca.
“It has not been concluded that there is any link between the COVID-19 Vaccine AstraZeneca and these cases.
“However, acting on the precautionary principal, and pending receipt of further information, the NIAC has recommended the temporary deferral of the COVID-19 vaccine AstraZeneca vaccination programme in Ireland.
“The NIAC is due to meet again this morning. A further statement will follow thereafter.”