A mind-blowing, small, soft, flexible water-soluble implant numbs nerves and blocks pain signals to the brain.
Researchers from Northwestern University have created a tiny, soft, flexible implant that can be used to instantly and drug-freely reduce pain. The first-of-its-kind device might offer a much-needed substitute for opioids and other drugs with a high potential for addiction.
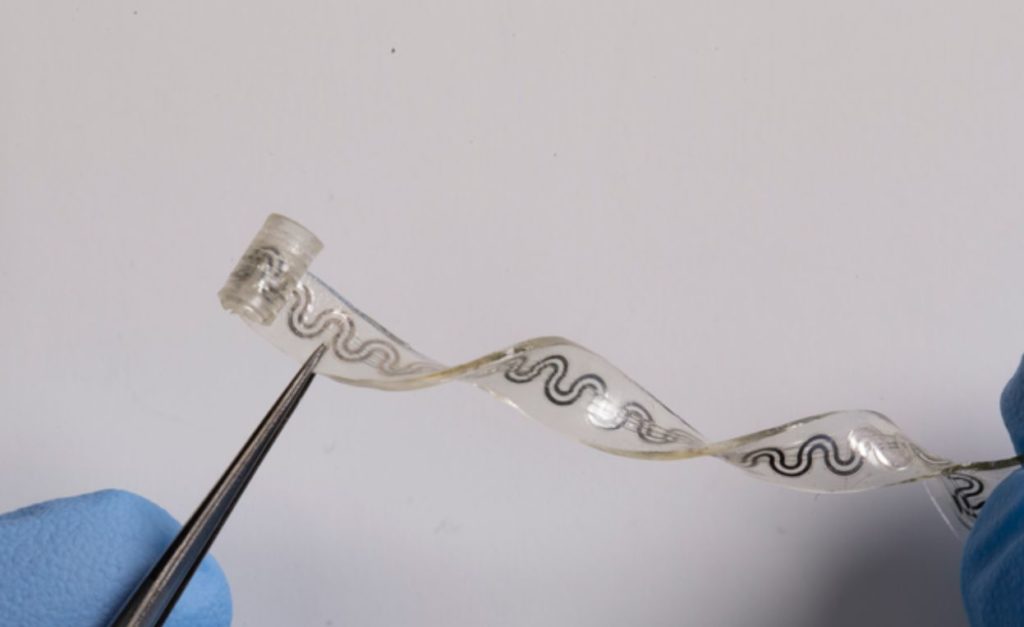
The biocompatible, water-soluble gadget numbs neurons and prevents pain signals from reaching the brain by gently wrapping around nerves and delivering precise, targeted cooling. The user can remotely activate the device and alter its intensity using an external pump. Without the need for surgical extraction, the gadget simply dissolves into the body when it is no longer required.
The researchers believe the tool has the greatest potential to benefit patients undergoing regular surgeries or even amputations, which frequently necessitate post-operative drugs. To assist in controlling the patient’s post-operative discomfort, surgeons could implant the device during the process.
The work is scheduled to appear in the July 1 edition of Science. The paper outlines the design of the device and illustrates its effectiveness using an animal model.
John A. Rogers of Northwestern University, who oversaw the creation of the device, stated that while opioids are quite effective, they are also very addictive. “As engineers, we are motivated by the idea of treating pain without drugs — in ways that can be turned on and off instantly, with user control over the intensity of relief. The technology reported here exploits mechanisms that have some similarities to those that cause your fingers to feel numb when cold. Our implant allows that effect to be produced in a programmable way, directly and locally to targeted nerves, even those deep within surrounding soft tissues.”
Rogers, a pioneer in bioelectronics, is the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering, and Neurological Surgery at Northwestern University Feinberg School of Medicine and McCormick School of Engineering. He founded the Querrey Simpson Institute for Bioelectronics and serves as its founding director. The paper’s first author is Jonathan Reeder, a former Ph.D. candidate in Rogers’ lab.
How things work
The new device may sound like science fiction, but it actually uses evaporation, a fundamental idea that everyone is familiar with. The device contains a liquid coolant that is stimulated to evaporate at the precise position of a sensory nerve, much to how sweat evaporates to cool the body.
According to study co-author Dr. Matthew MacEwan of Washington University School of Medicine in St. Louis, “As you cool down a nerve, the signals that travel through the nerve become slower and slower — eventually stopping completely.” “We are specifically targeting peripheral nerves, which connect your brain and your spinal cord to the rest of your body. These are the nerves that communicate sensory stimuli, including pain. By delivering a cooling effect to just one or two targeted nerves, we can effectively modulate pain signals in one specific region of the body.”
The cooling effect is caused by tiny microfluidic channels in the device. Perfluoropentane, a liquid coolant that is already clinically approved for use in pressurized inhalers and as an ultrasound contrast agent, is present in one channel. Dry nitrogen, an inert gas, is present in a separate tube. The reaction that takes place when the liquid and gas enter the same chamber causes the liquid to immediately evaporate. In addition, a tiny embedded sensor keeps track of the nerve’s temperature to prevent tissue damage from occurring if it gets too cold.
According to Rogers, excessive chilling can harm the nerve and the delicate tissues around it.
“The duration and temperature of the cooling must therefore be controlled precisely. By monitoring the temperature at the nerve, the flow rates can be adjusted automatically to set a point that blocks pain in a reversible, safe manner. On-going work seeks to define the full set of time and temperature thresholds below which the process remains fully reversible.”
Powerful precision
Although alternative cooling therapies and nerve blockers have been experimentally evaluated, they all have disadvantages that the novel device solves. In the past, researchers have looked into needle-injected cryotherapies, for instance. These imprecise methods chill wide swaths of the tissue rather than individual nerves, which may have unintended consequences like tissue injury and inflammation.
At its widest point, the small device from Northwestern is only 5 millimeters wide. Without the use of sutures, one end is twisted into a cuff that gently wraps around a single nerve. The technology spares neighboring areas from unneeded cooling, which could have negative effects, by carefully targeting only the damaged nerve.
“You don’t want to inadvertently cool other nerves or the tissues that are unrelated to the nerve transmitting the painful stimuli,” MacEwan added. “We want to block the pain signals, not the nerves that control motor function and enables you to use your hand, for example.”
Previous studies have also looked into nerve blockers that mute painful inputs via electrical stimulation. These also have restrictions.
Electrical stimulation cannot effectively shut down a nerve without first activating it, according to MacEwan.
“That can cause additional pain or muscle contractions and is not ideal, from a patient’s perspective.”
Disappearing act
This novel technology is the third example of bioresorbable electronic devices reported in Science by the Rogers team, which developed the notion of transient electronics in 2012.
In a paper published in Nature Medicine in 2018, Rogers, MacEwan, and colleagues demonstrated the first bioresorbable electronic device ever created, a biodegradable implant that accelerates nerve regeneration.
Then, in 2021, a transitory pacemaker was shown by Rogers and colleagues and published in Nature Biotechnology.
The device’s whole composition is biocompatible and will gradually and naturally dissolve into the body’s biofluids over the course of a few days or weeks without the need for surgical removal. Similar to absorbable stitches, the bioresorbable devices are fully safe.
The soft, flexible nerve cooling device is the best way to treat nerves that are as thin as a sheet of paper.
“If you think about soft tissues, fragile nerves and a body that’s in constant motion, any interfacing device must have the ability to flex, bend, twist and stretch easily and naturally,” Rogers added. “Furthermore, you would like the device to simply disappear after it is no longer needed, to avoid delicate and risky procedures for surgical removal.”
Image Credit: Getty
You were reading: Scientists create a New Device that Relieves Pain Without Drugs