Two blood pressure drugs are being recalled for possibly containing high levels of N-nitrosoirbesartan – a substance that can cause cancer, according to FDA website.
A voluntary recall of Irbesartan and hydrochlorothiazide pills is being made by Lupin Pharmaceuticals Inc. at the consumer level.
Based on laboratory test results, several tested API batches exceeded the specification limit for the impurity, N-nitrosoirbesartan — a potential human carcinogen (a substance that may cause cancer). Lupin is recalling all batches of Irbesartan Tablets USP 75mg, 150mg, and 300mg, as well as Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg, in the United States.
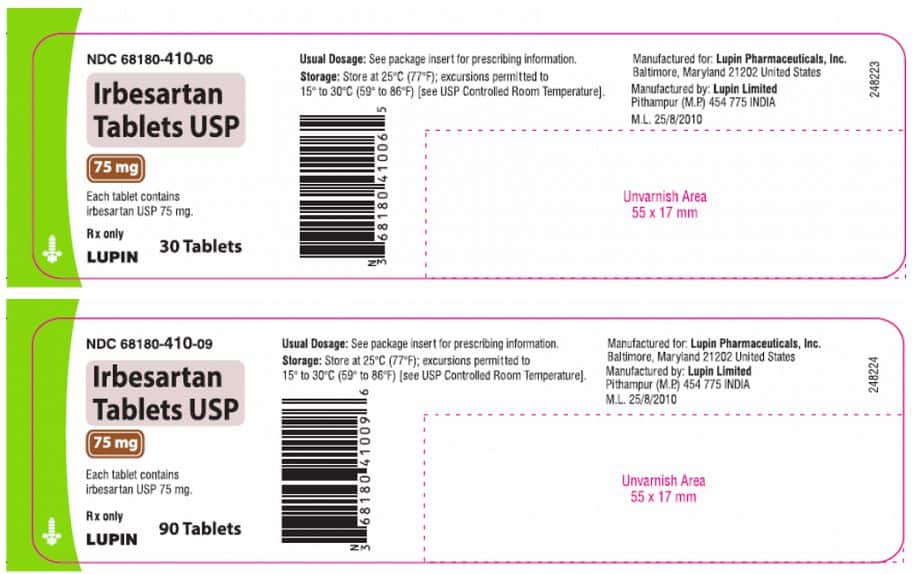
Irbesartan Tablets USP, 75mg, 150mg, and 300mg, are packed in 30- and 90-count bottles and delivered to wholesalers, drug chains, mail-order pharmacies, and supermarkets in the United States.
Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg, are packed in 30- and 90-count bottles and sold to wholesalers, drug chains, mail order pharmacies, and supermarkets across the United States.
According to the recall notice, the company is alerting its wholesalers, distributors, drug chains, mail-order pharmacies, and supermarkets by phone and by recall notification, and is arranging for the return of all recalled product lots.
Patients who are taking the drugs are urged to continue taking them and to contact their pharmacist, physician, or medical provider for information on other treatment options.
Inmar Rx Solutions Inc. can be reached at 855-769-3988 or 855-769-3989 if consumers, wholesalers, distributors, or retailers have any queries about this recall. Return the recalled lots to Inmar Rx Solutions, Inc. for reimbursement; the lot number can be located on the side of the bottle label.
Image Credit: Getty / FDA
You were reading: FDA: Lupin recalls two blood pressure drugs after finding cancer-causing substance