Decoding the Secrets of an Age-Related Enzyme and Its Interaction with Genetic Material
Recent research sheds light on the way an enzyme, involved in aging and metabolic regulation, interacts with our genetic content to control gene expression within cells.
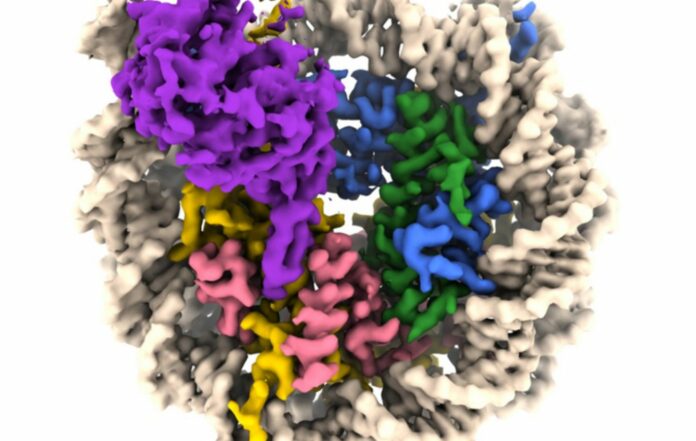
A group of researchers, spearheaded by a Penn State team, have captured images of a sirtuin enzyme linked to a nucleosome—a densely packed structure composed of DNA and histone proteins—demonstrating the enzyme’s ability to maneuver the nucleosome assembly and access both DNA and histone proteins, thereby elucidating its role in humans and other organisms.
The findings are detailed in a paper published today in the journal Science Advances.
Sirtuins represent a class of enzymes present in life forms from bacteria to humans, with crucial functions in aging, DNA damage detection, and tumor suppression in multiple cancers. Owing to these diverse roles, the pharmaceutical industry is investigating their potential in biomedical applications. A significant focus has been on the capacity of certain sirtuins to reduce gene expression by eliminating a chemical marker from histone proteins.
“In our cells,” as explained by author Song Tan, “DNA is not naked like we see it in textbooks; it is spooled around proteins called histones within a large complex called the nucleosome.”
The arrangement of this packaging can also influence gene activation or suppression: introducing an ‘acetyl’ chemical marker to the histone packaging material activates a gene, while removing the acetyl marker deactivates it. Sirtuins can mute gene activity by taking away the acetyl marker from histones within nucleosomes.
“Understanding how sirtuins interact with the nucleosome to remove this flag could inform future drug discovery efforts.”
Prior research has primarily examined how sirtuins engage with small sections of histones individually, mainly because such histone “tail” peptides are considerably simpler to handle in a laboratory setting. Tan explains that nucleosomes are a hundred times larger than the typical histone peptides used in these investigations, making them far more complex to work with.
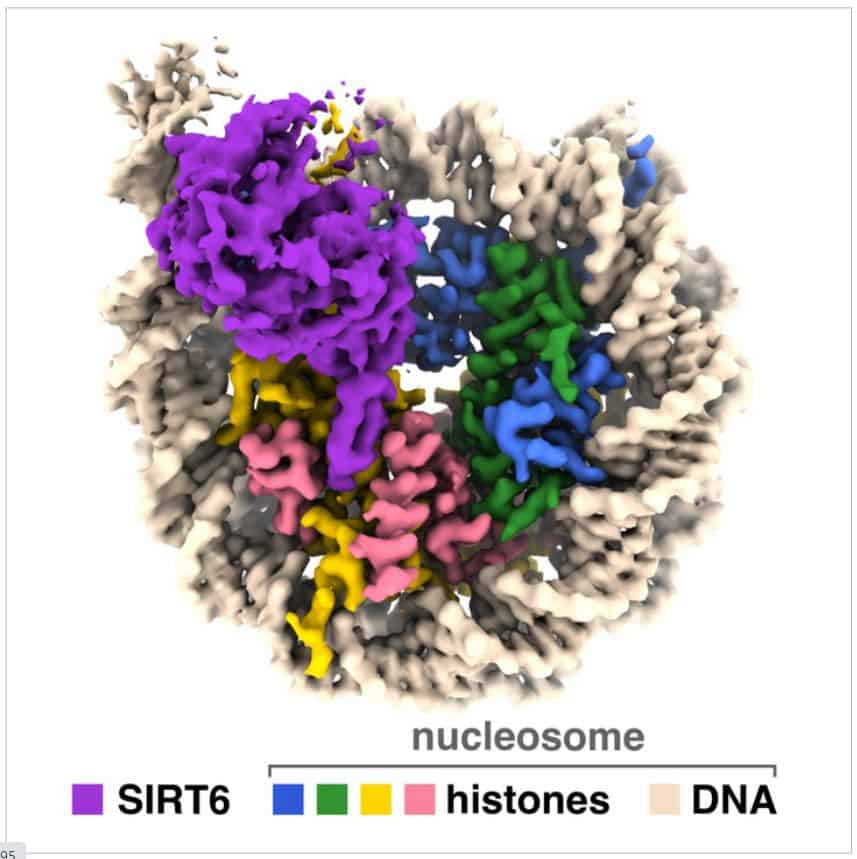
“We have visualized a sirtuin enzyme called SIRT6 on its physiologically relevant substrate—the entire nucleosome,” adds author Jean-Paul Armache.
They discovered that “SIRT6 interacts with multiple parts of the nucleosome, not only the histone where the acetyl flag is to be modified.”
Utilizing a sophisticated imaging technique called cryo-electron microscopy, with equipment at the Penn State Cryo-Electron Microscopy Facility, the National Cancer Institute, and the Pacific Northwest Cryo-EM Center, the researchers pinpointed the way SIRT6 positions itself on the nucleosome to remove an acetyl group from the K9 location on the H3 histone. The team collaborated with Craig Peterson’s lab at the University of Massachusetts Chan Medical School to conduct biochemical experiments that corroborated their findings.
The researchers discovered that SIRT6 attaches to the nucleosome through a connection known as an “arginine anchor.” This type of binding, first described by Tan’s lab in 2014, is employed by various proteins that target a notably acidic region on the nucleosome’s surface. In this instance, an extended loop—a structural feature of SIRT6—fits snugly into a depression in the acidic patch, much like a pipe resting in a trench.
“The arginine anchor is a common paradigm for how many chromatin proteins interact with the nucleosome,” adds Tan. “When we mutated the SIRT6 arginine anchor, the activity at the K9 position was severely affected, supporting a critical role for the SIRT6’s arginine anchor. Surprisingly, this mutation also impacted SIRT6’s enzymatic activity at a different position, K56, located much further away.”
Rather than SIRT6 attaching to the nucleosome using two distinct methods to reach the two separate histone locations, it seems that SIRT6 might bind in a manner that allows access to K9, and simultaneously provides access to K56.
“SIRT6 binds to a partially unwrapped nucleosome, with DNA displaced from the end of the nucleosome” adds Armache.
This revelation unveils the K56 position, suggesting that SIRT6 might be able to essentially extend itself to access that specific location.
In the future, the researchers aim to confirm this hypothesis. They also hope to investigate how SIRT6 cooperates with other enzymes and gain a deeper understanding of its function in the DNA damage response process.
Source: 10.1126/sciadv.adf7586
Image Credit: Song Tan Lab, Penn State