A Weird-looking Animal Unlocks the Secret to Immortality
There’s an unsettling reality that shadows human existence: the inexorable fact that we will all face the end of life.
This unavoidable truth has ignited many fantasies of immortal life, of being able to live for centuries more.
Although we humans are still grappling with the constraints of our finite lives, a number of creatures in the animal kingdom appear to be challenging the natural order, seemingly resisting the certainty of death.
A remarkable breakthrough concerning rejuvenation and aging was brought to light by researchers from the National Institutes of Health and their collaborators. They delved into the astonishing ability of a minuscule marine organism to spawn a whole new body from nothing but its oral structure.
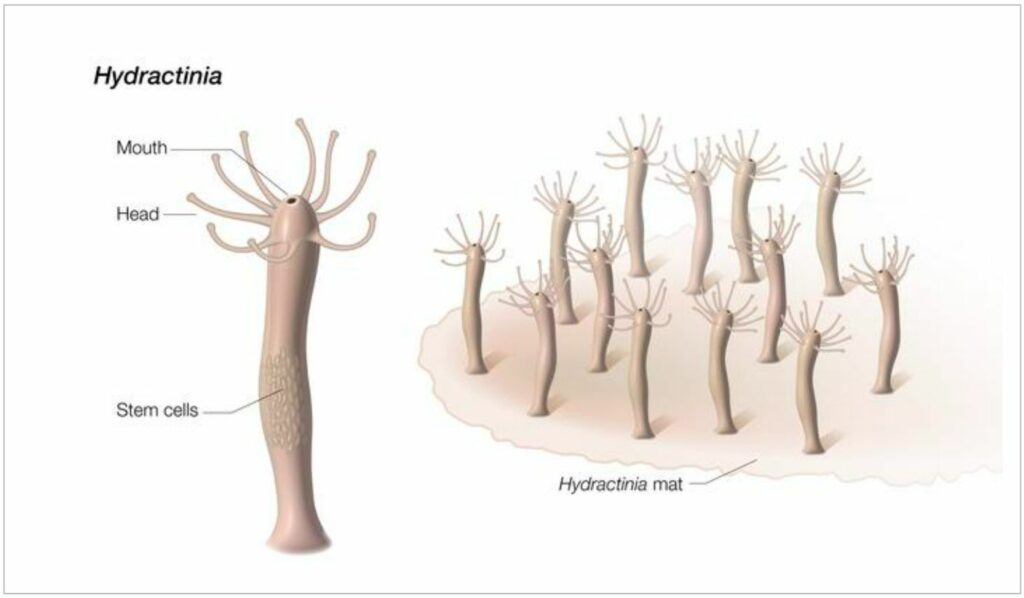
The scientists analyzed RNA sequences from Hydractinia symbiolongicarpus, a tiny tubular creature that makes its home on hermit crab shells. As the Hydractinia embarked on their astounding regenerative journey, the scientists spotted a molecular pattern akin to senescence, the biological progression of aging.
The research, disclosed in Cell Reports, unveils that in Hydractinia, the core biological mechanisms of regeneration and aging are inextricably linked, offering fresh insights into the evolution of aging.
“Studies like this that explore the biology of unusual organisms reveal both how universal many biological processes are and how much we have yet to understand about their functions, relationships and evolution,” points out Charles Rotimi, Ph.D., director of the Intramural Research Program at the National Human Genome Research Institute (NHGRI), part of NIH. “Such findings have great potential for providing novel insights into human biology.”
Deciphering the evolutionary beginnings of core biological phenomena, such as aging and regeneration, is crucial for comprehending human health and illness. Humans possess a degree of regenerative capabilities, such as mending a fractured bone or even reconstituting a harmed liver. However, certain animals, like salamanders and zebrafish, boast the ability to regenerate entire limbs and restore various organs. Interestingly, creatures with simpler anatomical structures, like the Hydractinia, frequently display the most astonishing regenerative capacities, which include the ability to develop an entirely new body from a fragment of tissue.
The concept of senescence playing a role in regeneration contrasts with observations in human cells.
“Most studies on senescence are related to chronic inflammation, cancer and age-related diseases,” remarks Andy Baxevanis, Ph.D., senior scientist at NHGRI and an author of the study.
“Typically, in humans, senescent cells stay senescent, and these cells cause chronic inflammation and induce aging in adjacent cells. From animals like Hydractinia, we can learn about how senescence can be beneficial and expand our understanding of aging and healing.”
In earlier studies, it was discovered that Hydractinia possesses a distinct set of stem cells dedicated to regeneration. Stem cells hold the remarkable ability to morph into different cell types, making them invaluable in forming new body parts. In humans, stem cells predominantly play a role during development, whereas highly regenerative organisms like Hydractinia utilize stem cells throughout their existence.
These regenerative stem cells in Hydractinia are stored in the lower part of the body. Intriguingly, even when the mouth — a region quite distant from where the stem cells are located — is removed, it is capable of growing a whole new body.
Unlike human cells, which are confined to their specific roles, the adult cells in some highly regenerative organisms can revert back to stem cells upon injury, though this mechanism remains enigmatic. The researchers hypothesized that Hydractinia must be producing new stem cells and began looking for molecular signals guiding this process.
When RNA sequencing led the researchers to senescence, they analyzed the Hydractinia genome for sequences akin to human senescence-associated genes. Out of three genes they pinpointed, one was activated in cells close to the area where the creature was cut. Upon deletion of this gene, the formation of senescent cells was hindered and, in the absence of these senescent cells, the organisms were unable to produce new stem cells and couldn’t regenerate.
To understand how Hydractinia avoids the detrimental effects of senescence, the researchers monitored the senescent cells within the creature. Astonishingly, they observed the animal expelling these cells through its mouth. While humans do not have the capacity to eliminate aging cells with such ease, the functions of senescence-related genes in Hydractinia offer insights into the evolution of the aging process.
Hydractinia and its close kin, such as jellyfish and corals, parted ways from a common ancestor with humans over 600 million years ago, and these creatures do not age in the conventional sense. Given these factors, Hydractinia can offer invaluable understanding regarding the early animal ancestors. As such, the researchers postulate that regeneration could have been the primary purpose of senescence in early animal life.
“We still don’t understand how senescent cells trigger regeneration or how widespread this process is in the animal kingdom,” adds Dr. Baxevanis.
“Fortunately, by studying some of our most distant animal relatives, we can start to unravel some of the secrets of regeneration and aging — secrets that may ultimately advance the field of regenerative medicine and the study of age-related diseases as well.”
Source: 10.1016/j.celrep.2023.112687
Image Credit: Shutterstock
