A breakthrough in dementia care? new mouse study shows lowering a form of brain cholesterol may reduce brain damage in Alzheimer’s and related dementias.
Alzheimer’s disease and similar dementias often involve a marked decline in cognitive abilities, a process linked to an excessive buildup of a standard brain protein, tau.
This accumulation of tau is associated with the deterioration and eventual death of surrounding brain tissue.
Recent findings from scientists at Washington University School of Medicine in St. Louis have shed new light on this process through studies conducted in mice.
They discovered that deposits of tau in the brain, similar to those seen in Alzheimer’s, result in increased levels of a specific type of cholesterol, termed cholesteryl esters.
Crucially, their research indicates that reducing these cholesteryl ester levels can play a significant role in averting both neurological damage and alterations in behavior.
Senior author David M. Holtzman, MD, and his team emphasize, “This has important therapeutic implications. The compound we used in this study has side effects that make it unsuitable for use in people. But if you could develop a therapy that reduces cholesteryl esters inside brain cells without unacceptable side effects, it would be a promising candidate to test in neurodegenerative diseases.”
Published in the journal Neuron, the study explored the role of the APOE gene, the primary genetic risk factor for Alzheimer’s, which is involved in cholesterol transport and immune cell activation.
The connection between cholesterol levels and dementia might be closer than previously understood. The primary genetic marker for increased risk of Alzheimer’s disease is the APOE gene, which plays a crucial role in the activation of the brain’s immune cells. Misactivation of these cells can lead to brain tissue damage.
Additionally, APOE is responsible for transporting cholesterol and other fats in the bloodstream, a function that is also implicated in the development of atherosclerosis.
Dr. David Holtzman, along with Dr. Alexandra Litvinchuk, a postdoctoral researcher, explored how APOE interacts with lipids and the resulting impact on brain health. They focused on mice engineered to possess a tau gene variant that makes them prone to tau protein accumulation, leading to neurodegenerative conditions.
These mice begin to show neurodegenerative symptoms by six months, with significant brain deterioration evident by nine and a half months, impairing their ability to perform standard activities like nest building.
The study involved mice with either a human version of the APOE gene, APOE3, which is associated with a standard Alzheimer’s risk, or APOE4, which significantly increases the disease’s risk, or with the APOE gene completely absent.
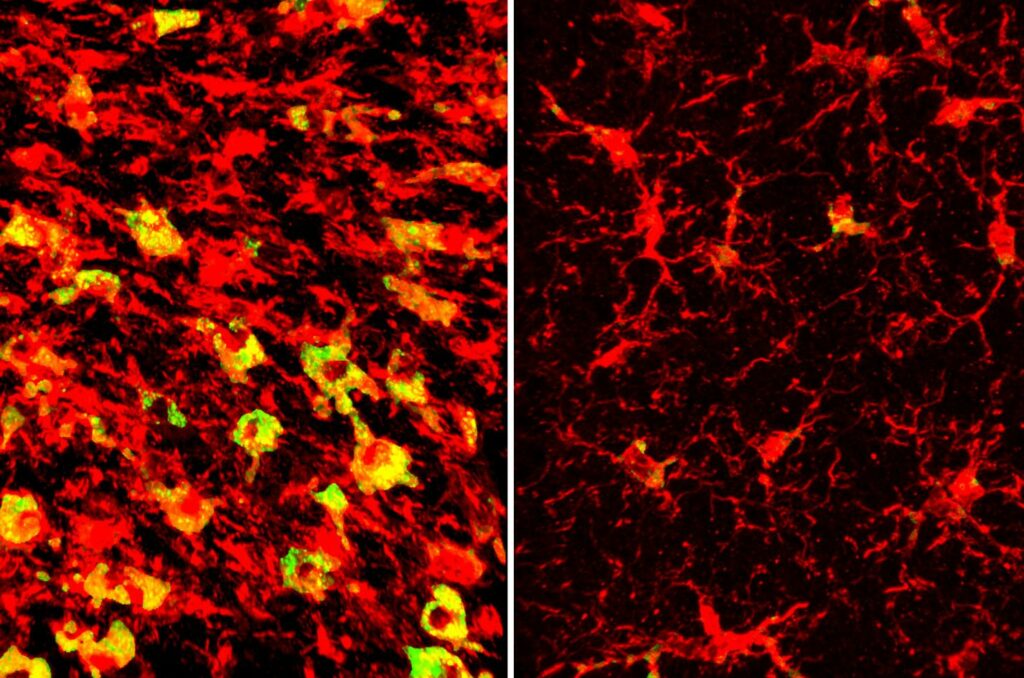
Their research found that the presence of APOE4 correlates with abnormal lipid metabolism in the brain. Notably, at nine and a half months, mice with the APOE4 gene showed not just brain tissue atrophy but also a peculiar excess of various lipids, with over 180 types affected.
One of the most prominent observations was that microglial cells—immune cells within the brain—were excessively filled with cholesteryl esters, unlike in the presence of APOE3. This lipid profiling was conducted in partnership with Denali Therapeutics and overseen by scientist Gilbert Di Paolo, PhD.
“Microglia filled up with lipids become hyperinflammatory and start secreting things that are not good for the brain,” Holtzman points out.
Thus, the removal of lipids from the brain might be a key factor in diminishing both inflammation and the progression of neurodegenerative diseases. The team tested this hypothesis using an LXR agonist, part of a new class of experimental drugs designed to reduce cell lipid levels.
They administered GW3965, the LXR agonist, to mice genetically modified to express the APOE4 gene from the age of six months. When these mice reached nine and a half months old—a stage where they would typically show severe brain damage—those treated with GW3965 exhibited a greater retention of brain volume compared to those given a placebo.
Treated mice also displayed reduced tau protein levels, decreased presence of inflammatory cells and inflammation, fewer synaptic losses, and improved nest-building abilities.
Further analysis showed that the LXR agonist’s effectiveness stems from its ability to activate the Abca1 gene, which is instrumental in transporting cholesterol and other fats out of the cells. Techniques to genetically enhance Abca1 expression mirrored the beneficial effects of the LXR agonist treatment, leading to decreased lipid buildup, reduced tau levels, and less neurodegeneration.
“What’s exciting is that we see all these effects in an animal model that shares a lot of features with human neurodegenerative diseases,” remarks Holtzman. “It shows that this kind of approach could have a lot of promise.”
However, a significant challenge in adapting this treatment for human use is that LXR agonists can disrupt lipid metabolism in the liver, potentially causing fatty liver disease.
Researchers are currently seeking to create LXR agonists devoid of this adverse effect. Such advancements could not only aid in treating brain diseases but might also offer therapeutic benefits for heart disease.
Dr. Holtzman points out:“There’s a lot of similarity between the mechanism that’s driving immune cells to damage the brain in Alzheimer’s disease and the one that’s driving the same kinds of immune cells to cause vascular damage in atherosclerosis. In both cases, lipids accumulate in immune cells, causing them to become hyperinflammatory and damage nearby tissues. Getting rid of that lipid accumulation may have double benefits for human health.”
Image Credit: iStock
