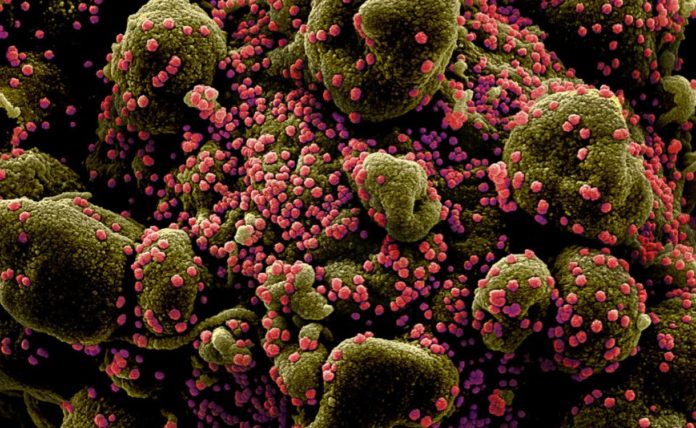
The UK has become the first country in the world to allow volunteer exposure to the COVID-19 virus for medical research. Up to 90 healthy volunteers between the ages of 18 and 30 will be infected with the virus in a safe and controlled environment.
The researchers have invited healthy young people, who are at lower risk of complications from the coronavirus, to volunteer for the study. Participants will receive compensation for time and all possible health protection. The British Government has allocated $46.6 million to the project.
- Scientists in Fear of This New Predator From Red Sea Eating Native Species in Mediterranean
- Does This Mean We Stopped Being Animal and Started Being Human Due to ‘Copy Paste’ Errors?
- The One Lifestyle Choice That Could Reduce Your Heart Disease Risk By More Than 22%
- Aging: This Is What Happens Inside Your Body Right After Exercise
- Immune-Boosting Drink that Mimics Fasting to Reduce Fat – Scientists ‘Were Surprised’ By New Findings
How does it work
One of the objectives of the study is to establish the smallest amount of virus necessary to cause infection, which is known as the characterization of the virus. It will also help clinicians understand how the immune system reacts to coronavirus and identify factors that influence the mode of transmission.
“The safety of volunteers is paramount, which means this virus characterisation study will initially use the version of the virus that has been circulating in the UK since March 2020 and has been shown to be of low risk in young healthy adults. Medics and scientists will closely monitor the effect of the virus on volunteers and will be on hand to look after them 24 hours a day,” assures the Government of the country.
Once the initial study is done, the volunteers could participate in its second phase, where they will be administered one of the approved vaccines to identify the most effective one. These types of trials play a critical role in the development of effective vaccines and treatments.
“Over many decades, these studies have been conducted safely and have played an important role in accelerating the development of treatments for diseases such as malaria, typhoid, cholera, norovirus, and influenza,” says the British Government.
As for the World Health Organization, its rules indicate that human trials (human challenge studies) are ethical when they meet certain criteria. Participants in the trial must be relatively young and in good health, provided with the highest quality medical care with frequent follow-up.
- Scientists in Fear of This New Predator From Red Sea Eating Native Species in Mediterranean
- Does This Mean We Stopped Being Animal and Started Being Human Due to ‘Copy Paste’ Errors?
- The One Lifestyle Choice That Could Reduce Your Heart Disease Risk By More Than 22%
- Aging: This Is What Happens Inside Your Body Right After Exercise
- Immune-Boosting Drink that Mimics Fasting to Reduce Fat – Scientists ‘Were Surprised’ By New Findings
The UK suffers from the spread of several variants of the virus after a strain that emerged in the country late last year became dominant and was detected in more than 80 countries around the world. However, so far, preliminary studies have shown that current vaccines are still effective against these new strains.